Thia-crown ether
In organic chemistry, thia-crown ethers are organosulfur compounds which are the thia analogues of crown ethers (cyclic polyethers). That is, they have a sulfur atom (sulfide linkage, −S−) in place of each oxygen atom (ether linkage, −O−) around the ring. While the parent crown ethers have the formulae (CH2CH2O)n, the parent thia-crown ethers have the formulae (CH2CH2S)n, where n = 3, 4, 5, 6. They have trivial names "x-ane-Sy", where x and y are the number of atoms in the ring and the number of those atoms that are sulfur, respectively. Thia-crown ethers exhibit affinities for transition metals.
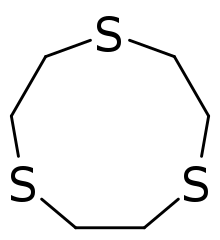
1,4,7-Trithiacyclononane (9-ane-S3) is a tridentate ligand and forms complexes with many metal ions, including those considered hard, such as copper(II) and iron(II).[1]
Tetradentate 14-ane-S4[2] and the hexadentate 18-ane-S6[3] are also known.
_dication.png.webp)
References
- Kueppers, H. J.; Wieghardt, K.; Nuber, B.; Weiss, J. W.; Bill, E.; Trautwein, A. X. (1987). "Crown Thioether Chemistry of Iron(II/III). Synthesis and Characterization of Low-spin Bis(1,4,7-trithiacyclononane)iron(III) and crystal structure of [FeII([9]aneS3)([9]aneS3(O))](ClO4)2•2NaClO4•H2O". Inorganic Chemistry. 26 (22): 3762–3769(8). doi:10.1021/ic00269a028.
- Prett, V; Diaddario, L; Dockal, E; Corfield, P; Ceccarelli, C; Glick, M; Ochrymowycz, L. A.; Rorabacher, D. B. (1983). "Ring size effects on the structure of macrocyclic ligand complexes: copper(II) complexes with 12–16-membered cyclic tetrathia ethers". Inorganic Chemistry. 22 (24): 3661–3670. doi:10.1021/ic00166a033.
- Shaw, J; Wolowska, J; Collison, D; Howard, J; McInnes, E; McMaster, J; Blake, A; Wilson, C; Schroeder, M (2006). "Redox Non-innocence of Thioether Macrocycles: Elucidation of the Electronic Structures of Mononuclear Complexes of Gold(II) and Silver(II)". Journal of the American Chemical Society. 128 (42): 13827–13839. doi:10.1021/ja0636439. PMID 17044711.