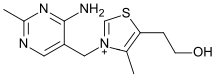
Thiaminase
Thiaminase is an enzyme that metabolizes or breaks down thiamine into pyrimidine and thiazole. It is an antinutrient when consumed.
| Thiamine pyridinylase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| EC no. | 2.5.1.2 | ||||||||
| CAS no. | 9030-35-7 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| |||||||||
| Aminopyrimidine aminohydrolase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| EC no. | 3.5.99.2 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
The old name was "aneurinase".[1]
There are two types:[2]
- Thiamine pyridinylase, Thiaminase I (EC 2.5.1.2, InterPro: IPR030901)
- Produced by Clostridium thiaminolyticum (anaerobic organism that occurs in the human small intestine
- Aminopyrimidine aminohydrolase, Thinaminase II (EC 3.5.99.2, InterPro: IPR027574, IPR004305)
- Produced by Bacillus thiaminoluticum (aerobic organism that occurs in the human colon)
Enzyme Commission Number
Enzyme commission (EC) numbers were first implemented by the Nomenclature Committee of the International Union of Biochemistry and Molecular Biology (IUBMB). To be exact an enzyme commission number denotes a numerical classification of an enzyme gene as well as the enzyme. It is also used as an identifier of enzymatic reactions.[3] This number helps to identify enzyme-catalyzed reactions. When determining EC numbers there are six main levels to take into account: Oxidoreductase reactions, Transferase reactions, Hydrolase reactions, Lyase reactions, Isomerase reactions, and Ligase reactions.[3] To assign an enzyme and EC number it is performed manually based on experimental data that has been published on individual enzymes. For Thiaminase there are two different EC numbers for the two types. Thiaminase I has an EC number of 2.5.1.2 and Thiaminase II has an EC number of 3.5.99.2.[4]
Structure and Function
Enzyme Reaction Pathway
Thiaminase I works to cleave the pyrimidine ring in thiamin from the thiazolium ring at the methylene bridge.[4] From there it adds a base compound to the pyrimidine in order to create an analogue inhibitor of thiamin.[4] As for Thiaminase II it works in a similar way but does not add a base compound. Other differences between the two enzymes are the substrates used to split the C-N bond that connects the two heterocyclic rings in thiamin. Thiaminase I has the ability to use a multitude of substrates like cysteine, pyridine, aniline, veratrylamine, dithiothreitol, and quinoline. On the other hand, Thiaminase II can only use water.[4]
Thiaminase I
When analyzing the structure of Thiaminase I it shows a fold similar to that of group II periplasmic binding proteins like maltose-binding protein.[5] These periplasmic binding proteins have two domains that each contain an α/β fold. These two domains come together to form a deep cleft that are connected by three crossover segments. Due to this structure scientists proposed that Thiaminase I could have evolved from prehistoric periplasmic binding protein that had been responsible for up taking thiamin.[5] Between the two domains, in the cleft, sit the active site for Thiaminase I. Along the cleft there are four acidic residues and six tyrosine residues. In order for Thiamin to interact with Thiaminase I it is positioned in the active site between the pyrimidine and Asp272 by two hydrogen bonds. The Glu241 the goes on to activate the Cys113 to attack C6 of the pyrimidine. This forms a zwitterionic intermediate.[5] The Glu241 causes and protonation and nucleophilic attack that results in the split of the bond between the pyrimidine and the thiazole. When observing the crystalline structure, it has two ⍺/ꞵ-type domains separated by a large cleft. At room temperature the two molecules have a noncrystallographic twofold axis that are bridged by a sulfate ion. [6]
Thiaminase II
Thiaminase II has been found to be TenA. In order to cleave the C-N bond between the thiazole and pyrimidine Thiaminase only uses water as its nucleophile. When viewing Thiaminase II it is found to have a crystal structure that has 11 helices surrounding a deep acidic pocket.[5] For each monomer present in the quaternary structure it interacts with two other monomers. There are several residues like Tyr112, Phe208, Tyr47, and Tyr163 that have some sort of contribution to the π- stacking environment surrounding the HMP ligand.[5] The Glu205 side chain will form a hydrogen bond with the N1 nitrogen in the pyrimidine ring. Next the Tyr163 and the Asp44 side chain come together to form the hydrogen bonds with the N3 and N4'.[5] Finally the Cys135 catalytic residue is positioned near the C2 in the pyridine ring to complete the split of thiamin into its heterocycles.[5]
Sources
This enzyme can be found in a variety of different sources. It can be found in marine organisms, plants, and bacteria. Since Thiamine (vitamin B1) is a very important substance required for metabolic pathways by almost all organisms, it can be very detrimental to introduce Thiaminase to a system. Frequently an organism gains this enzyme by ingesting another organism that carries it. In most cases, prey fish will contain one of the bacteria that produces this enzyme. When that prey fish is consumed raw without treatment the bacteria will transfer to the consumer.[7] The consumer eventually will fall ill then parish from a Thiamine deficiency. This has been seen in different lab studies. Through these studies the enzyme has been found in zebra fish as well as red cornet fish.[7] Thiaminase is also present in other sources as well.
Sources include:
Effects
Its physiological role for fish, bacterial cell or insect is not known. However, in ferns it is thought to offer protection from insects[14] while studies have shown that thiamine hydrolase (thiaminase type 2) which was originally thought to be involved solely in the degradation of thiamine has actually been identified as having a role in thiamine degradation with the salvage of the pyrimidine moiety where thiamin hydrolysis product N-formyl-4-amino-5-aminomethyl-2-methylpyrimidine is transported into the cell and deformylated by the ylmB-encoded amidohydrolase and hydrolyzed to 5-aminoimidazole ribotide.[15]
It was first described in 1941 as the cause of highly mortal ataxic neuropathy in fur-producing foxes eating raw entrails of river fish like carp.
It is also known as the cause of cerebrocortical necrosis of cattle and polioencephalomalasia of sheep eating thiaminase containing plants.[16][17]
It was once causing economical losses in raising fisheries, e.g. in yellowtail fed raw anchovy as a sole feed for a certain period, and also in sea bream and rainbow trout. The same problem is being studied in a natural food chain system.[18]
The larvae of a wild silk worm Anaphe venata are being consumed in a rain forest district of Nigeria as a supplemental protein nutrition, and the heat-resistant thiaminase in it is causing an acute seasonal cerebellar ataxia named African seasonal ataxia or Nigerian seasonal ataxia.[19]
In 1860–61, Burke and Wills were the first Europeans to cross Australia south to north; on their return they subsisted primarily on raw nardoo-fern. It is possible that this led to their death due to the extremely high levels of thiaminase contained in nardoo. The Aborigines prepared nardoo by soaking the sporocarps in water for at least a day to avoid the effects of thiamine deficiency that would result from ingesting the leaves raw. In the explorers' journals they noted many symptoms of thiamine deficiency, so it is thought that they did not soak the nardoo long enough. Eventually thiamine deficiency could have led to their demise. It is noteworthy to mention that there are several other hypotheses regarding what may have killed Burke and Wills and it is widely disagreed upon by historians and scientists alike.[2]
References
- Fujita A, Nose Y, Kozuka S, Tashiro T, Ueda K, Sakamoto S (May 1952). "Studies on thiaminase. I. Activation of thiamine breakdown by organic bases". The Journal of Biological Chemistry. 196 (1): 289–95. PMID 12980969.
- Thiaminases
- Hu, Qian-Nan; Zhu, Hui; Li, Xiaobing; Zhang, Manman; Deng, Zhe; Yang, Xiaoyan; Deng, Zixin (2012-12-28). "Assignment of EC Numbers to Enzymatic Reactions with Reaction Difference Fingerprints". PLoS ONE. 7 (12): e52901. doi:10.1371/journal.pone.0052901. ISSN 1932-6203. PMC 3532301. PMID 23285222.
- "Thiaminase - an overview | ScienceDirect Topics". www.sciencedirect.com. Retrieved 2023-10-24.
- Jurgenson, Christopher T.; Begley, Tadhg P.; Ealick, Steven E. (2009). "The Structural and Biochemical Foundations of Thiamin Biosynthesis". Annual review of biochemistry. 78: 569–603. doi:10.1146/annurev.biochem.78.072407.102340. ISSN 0066-4154. PMC 6078420. PMID 19348578.
- Campobasso, Nino; Costello, Colleen A.; Kinsland, Cynthia; Begley, Tadhg P.; Ealick, Steven E. (1998-11-01). "Crystal Structure of Thiaminase-I from Bacillus t hiaminolyticus at 2.0 Å Resolution ,". Biochemistry. 37 (45): 15981–15989. doi:10.1021/bi981673l. ISSN 0006-2960.
- Richter, Catherine A.; Evans, Allison N.; Heppell, Scott A.; Zajicek, James L.; Tillitt, Donald E. (2023-01-13). "Genetic basis of thiaminase I activity in a vertebrate, zebrafish Danio rerio". Scientific Reports. 13 (1): 698. doi:10.1038/s41598-023-27612-5. ISSN 2045-2322.
- NcCleary BV, Chick BF (1977). "The purification and properties of a thiaminaseI from Nardoo (Marsilea drummondii)". Phytochemistry. 16 (2): 207–213. doi:10.1016/S0031-9422(00)86787-4.
- Boś M, Kozik A (February 2000). "Some molecular and enzymatic properties of a homogeneous preparation of thiaminase I purified from carp liver". Journal of Protein Chemistry. 19 (2): 75–84. doi:10.1023/A:1007043530616. PMID 10945431.
- Wittliff JL, Airth RL (February 1968). "The extracellular thiaminase I of Bacillus thiaminolyticus. I. Purification and physicochemical properties". Biochemistry. 7 (2): 736–44. doi:10.1021/bi00842a032. PMID 4966932.
- Nakatsuka T, Suzuki K, Nakano Y, Kitaoka S (1988). "Physicochemical properties of intracellular thiaminase II of Bacillus aneurinolyticus". Vitamins (Japan). 62: 15–22.
- Toms AV, Haas AL, Park JH, Begley TP, Ealick SE (February 2005). "Structural characterization of the regulatory proteins TenA and TenI from Bacillus subtilis and identification of TenA as a thiaminase II". Biochemistry. 44 (7): 2319–29. doi:10.1021/bi0478648. PMID 15709744.
- Nishimune T, Watanabe Y, Okazaki H, Akai H (2000). "Thiamin is decomposed due to Anaphe spp. entomophagy in seasonal ataxia patients in Nigeria". J. Nutr. 130: 1625–28.
- Vetter J (2010). "Toxicological and Medicinal Aspects of the Most Frequent Fern Species, Pteridium aquilinum (L.) Kuhn". Working with Ferns: Issues and Applications. pp. 361–375. doi:10.1007/978-1-4419-7162-3_25.
- Jenkins AH, Schyns G, Potot S, Sun G, Begley TP (August 2007). "A new thiamin salvage pathway". Nature Chemical Biology. 3 (8): 492–7. doi:10.1038/nchembio.2007.13. PMID 17618314.
- Ramos JJ, Marca C, Loste A, García de Jalón JA, Fernández A, Cubel T (February 2003). "Biochemical changes in apparently normal sheep from flocks affected by polioencephalomalacia". Veterinary Research Communications. 27 (2): 111–24. doi:10.1023/A:1022807119539. PMID 12718505.
- Evans WC (1975). "Thiaminases and their effects on animals". Vitamins and Hormones. 33: 467–504. doi:10.1016/S0083-6729(08)60970-X. ISBN 978-0-12-709833-3. PMID 779253.
- Fisher JP, Brown SB, Wooster GW, Bowser PR (December 1998). "Maternal blood, egg and larval thiamin levels correlate with larval survival in landlocked Atlantic salmon". The Journal of Nutrition. 128 (12): 2456–66. doi:10.1093/jn/128.12.2456. PMID 9868194.
- Adamolekun B, Adamolekun WE, Sonibare AD, Sofowora G (March 1994). "A double-blind, placebo-controlled study of the efficacy of thiamine hydrochloride in a seasonal ataxia in Nigerians". Neurology. 44 (3 Pt 1): 549–51. doi:10.1212/wnl.44.3_part_1.549. PMID 8145931.
External links
- thiaminase+I at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- thiaminase+II at the U.S. National Library of Medicine Medical Subject Headings (MeSH)