Thiazyl trifluoride
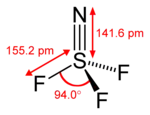
Thiazyl trifluoride is a chemical compound of nitrogen, sulfur, and fluorine, having the formula NSF3. It exists as a stable, colourless gas, and is an important precursor to other sulfur-nitrogen-fluorine compounds.[1] It has tetrahedral molecular geometry around the sulfur atom, and is regarded to be a prime example of a compound that has a sulfur-nitrogen triple bond.[2]
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Thiazyl trifluoride | |||
| Other names
Sulfur(VI) nitride trifluoride | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChEBI | |||
| ChemSpider | |||
PubChem CID |
|||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| NSF3 | |||
| Molar mass | 103.06 g·mol−1 | ||
| Appearance | Colourless gas | ||
| Melting point | −72.6 °C (−98.7 °F; 200.6 K) | ||
| Boiling point | −27.1 °C (−16.8 °F; 246.1 K) | ||
| Structure | |||
| Tetrahedral at the S atom | |||
| Hybridisation | sp3 | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |||
Preparation
NSF3 can be synthesised by the fluorination of thiazyl fluoride, NSF, with silver(II) fluoride, AgF2:
- NSF + 2 AgF2 → NSF3 + 2 AgF
or by the oxidative decomposition of FC(O)NSF2 by silver(II) fluoride:[3]
- FC(O)NSF2 + 2 AgF2 → NSF3 + 2 AgF + COF2
It is also a product of the oxidation of ammonia by S2F10.[4]
Reactions
NSF3 is much stable than thiazyl fluoride, does not reacts with ammonia and hydrogen chloride, and only reacts with sodium at 400 °C.[5] It reacts with carbonyl fluoride (COF2) in the presence of hydrogen fluoride to form pentafluorosulfanyl isocyanate (SF5NCO).[6]
References
- Oskar Glemser and Rüdiger Mews (1980). "Chemistry of Thiazyl Fluoride (NSF) and Thiazyl Trifluoride (NSF3): A Quarter Century of Sulfur-Nitrogen-Fluorine Chemistry". Angew. Chem. Int. Ed. Engl. 19 (11): 883–899. doi:10.1002/anie.198008831.
- Borrmann, T.; Lork, E.; Mews, R. D.; Parsons, S.; Petersen, J.; Stohrer, W. D.; Watson, P. G. (2008). "The crystal structures of NSF
3 and (NSF2N(CH3)CH2–)2: How short is the 'Crystallographic' N≡S triple bond?". Inorganica Chimica Acta. 361 (2): 479–486. doi:10.1016/j.ica.2007.05.016. - Chivers, Tristram; Laitinen, Risto S. (2006). "Chalcogen–Nitrogen Chemistry". In Devillanova, Francesco (ed.). Handbook of Chalcogen Chemistry. London: The Royal Society of Chemistry. p. 238. doi:10.1039/9781847557575. ISBN 978-0-85404-366-8.
- Steve Mitchell (1996). Steve Mitchell (ed.). Biological interactions of sulfur compounds. CRC Press. p. 14. ISBN 0-7484-0245-4.
- Huheey, James E.; Keiter, Ellen A.; Keiter, Richard L. (2003). Anorganische Chemie (in German). Berlin: Walter de Gruyter. p. 1021. ISBN 978-3-11-017903-3.
- US patent 3,666,784, Alan F. Clifford, Thomas C. Rhyne and James W. Thompson, "Process For Preparing .alpha.,.alpha.-Fluorinated Alkyl Isocyanates", issued 1972-05-30