Thioacyl chloride
In organic chemistry, thioacyl chloride is a functional group of the type RC(S)Cl, where R is an organic substituent. Thioacyl chlorides are analogous to acid chlorides, but much rarer and less robust. The best studied is thiobenzoyl chloride, a purple oil first prepared by chlorination of dithiobenzoic acid with a combination of chlorine and thionyl chloride.[1][2] A more modern preparation employs phosgene as the chlorinating agent,[3] this also generates carbonyl sulfide as a by-product.
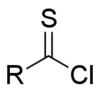
General structure of the thioacyl chloride functional group
PhCS2H + COCl2 → PhC(S)Cl + HCl + COS The most common thioacyl chloride is thiophosgene.
References
- Hermann Staudinger; Siegwart, J. (1920). "Ueber Thiobenzoylchlorid" [Thiobenzoyl chloride]. Helvetica Chimica Acta. 3: 824–833. doi:10.1002/hlca.19200030177.
- Potts KT, Sapino C (1972). "Thiocarbonyl halides". In Saul Patai (ed.). Acyl Halides. PATAI'S Chemistry of Functional Groups. pp. 349–380. doi:10.1002/9780470771273.ch11. ISBN 9780470771273.
- Viola H, Mayer R (1975). "Eine neue Darstellungsmethode für aromatische Thiocarbonsäurechloride" [A New Preparation Route for Aromatic Thiocarboxylic Acid Chlorides]. Z. Chem. 15 (9): 348. doi:10.1002/zfch.19750150904.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.