Thiocarbamate
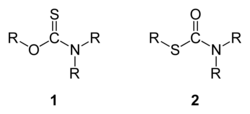
In organic chemistry, thiocarbamates (thiourethanes) are a family of organosulfur compounds. As the prefix thio- suggests, they are sulfur analogues of carbamates. There are two isomeric forms of thiocarbamates: O-thiocarbamates, ROC(=S)NR2 (esters), and S-thiocarbamates, RSC(=O)NR2 (thioesters).
Synthesis
Thiocarbamates can be synthesised by the reaction of water or alcohols upon thiocyanates (Riemschneider thiocarbamate synthesis):[1][2]
- RSCN + H2O → RSC(=O)NH2
- RSCN + R'OH → RSC(=O)NR'H
Similar reactions are seen between alcohols and thiocarbamoyl chlorides such as dimethylthiocarbamoyl chloride; as well as between thiols and cyanates.[2]
Alternatively, they arise by the reaction of amines with carbonyl sulfide.
- 2 R2NH + COS → [R2NH2+][R2NCOS−]
Reactions
In the Newman-Kwart rearrangement O-thiocarbamates can isomerise to S-thiocarbamates.[3] This reaction, which generally requires high temperatures, is an important method for the synthesis of thiophenols.

Dithiocarbamates
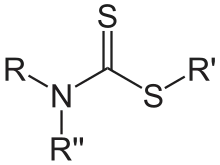
Dithiocarbamates are related to thiocarbamates by the replacement of O by S. Despite this structural similarity their synthesis and chemistry is quite different. Dithiocarbamates and their derivatives are widely used in the vulcanization of rubber.[4]
Borate,[5] Molybdenum, or Zinc[6] thiocarbamates are also used in metal-to-metal lubrication proposes, mainly as an anti-oxidation or anti-extreme pressure (EP) additive. 1-2% of such compounds can be added to internal combustion engine lubricant to increase extreme pressure performance in high operational temperatures.
See also
References
- Smith, Michael B.; March, Jerry (2007), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (6th ed.), New York: Wiley-Interscience, p. 1269, ISBN 978-0-471-72091-1
- Walter, W.; Bode, K.-D. (April 1967). "Syntheses of Thiocarbamates". Angewandte Chemie International Edition in English. 6 (4): 281–293. doi:10.1002/anie.196702811.
- Newman, Melvin S.; Hetzel, Frederick W. (1971). "Thiophenols from Phenols: 2-Naphthalenethiol". Org. Synth. 51: 139. doi:10.15227/orgsyn.051.0139.
- Engels, Hans-Wilhelm; et al. "Rubber, 4. Chemicals and Additives". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a23_365.pub2.
- Shah, Faiz Ullah. "Novel Alkylborate–Dithiocarbamate Lubricant Additives: Synthesis and Tribophysical Characterization".
- Shekarriz, M. "Investigation of antioxidant properties of Zinc and Molybdenum dithiocarbamates in hydrocarbons".