Thioenol
In organic chemistry, thioenols (also known as alkenethiols) are alkenes with a thiol group (−SH) affixed to one of the carbon atoms composing the double bond (i.e. C=C−SH). They are the sulfur analogs of enols (hence the thio- prefix). Alkenes with a thiol group on both atoms of the double bond are called enedithiols. Deprotonated anions of thioenols are called thioenolates.
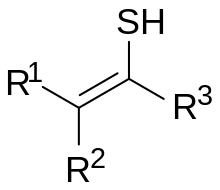
General chemical structure of a thioenol
These structures exhibit tautomerism to give thioketones or thioaldehydes, analogous to keto–enol tautomerism of carbonyl structures.[1]
References
- Chiang, Yvonne; Kresge, A. Jerry; Schepp, Norman P.; Popik, Vladimir V.; Rappoport, Zvi; Selzer, Tzvia (1998). "The acid dissociation constant of triphenylethenethiol, a simple thioenol, and that of its oxygen–enol analog". Canadian Journal of Chemistry. 76 (6): 657–661. doi:10.1139/v98-027.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.