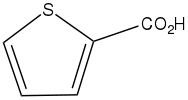
Thiophene-2-carboxylic acid
Thiophene-2-carboxylic acid is an organic compound with the formula SC4H3CO2H. It is one of two monocarboxylic acids of thiophene, the other being thiophene-3-carboxylic acid.[1] Copper(I) thiophene-2-carboxylate is a catalyst for Ullmann coupling reactions.
 | |
| Names | |
|---|---|
| Other names
2-thenoic acid; tenoic acid; Rhinotrophyl | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.007.659 |
| EC Number |
|
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C5H4O2S | |
| Molar mass | 128.15 g·mol−1 |
| Appearance | white solid |
| Melting point | 125–127 °C (257–261 °F; 398–400 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Synthesis
It can be prepared by the oxidation of thiophene-2-carboxaldehyde or, more practically, 2-acetylthiophene.[2]
Applications and reactions
Upon treatment with LDA, thiophene-2-carboxylic acid undergoes double deprotonation to give the 5-lithio derivative, a precursor to many 5-substituted derivatives.[3]
Thiophene-2-carboxylic acid has been widely studied as a substrate in coupling reactions and olefinations.[4][5]
References
- E. Campaigne, William M. LeSuer (1953). "3-Thenoic Acid". Organic Syntheses. 33: 94. doi:10.15227/orgsyn.033.0094.
- Swanston, Jonathan (2006). "Thiophene". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a26_793.pub2. ISBN 3527306730..
- Knight, David W.; Nott, Andrew P. (1983). "Generation and Synthetic Utility of Dianions Derived from Thiophenecarboxylic Acids". Journal of the Chemical Society, Perkin Transactions 1: 791-4. doi:10.1039/p19830000791.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Gooßen, Lukas J.; Deng, Guojun; Levy, Laura M. (2006). "Synthesis of Biaryls via Catalytic Decarboxylative Coupling". Science. 313 (5787): 662–664. Bibcode:2006Sci...313..662G. doi:10.1126/science.1128684. PMID 16888137. S2CID 1781760.
- Rakshit, Souvik; Grohmann, Christoph; Besset, Tatiana; Glorius, Frank (2011). "Rh(III)-Catalyzed Directed C−H Olefination Using an Oxidizing Directing Group: Mild, Efficient, and Versatile". Journal of the American Chemical Society. 133 (8): 2350–2353. doi:10.1021/ja109676d. PMID 21275421.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.