Thioketene
In organic chemistry, thioketenes are organosulfur compounds analogous to ketenes with the general formula R2C=C=S, where R is alkyl or aryl. The parent thioketene (ethenthione) is has the formula CH2=C=S. It is the simplest thioketene. Thioketene is stable as a gas, but like most thioketenes, it polymerizes upon condensation.[1]
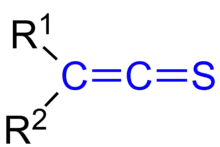
Some thioketenes are produced as transient species upon pyrolysis of 1,2,3-thiadiazoles.[2] It has been suggested that thioketene could be involved in cell damage processes.[3]
Isolable thioketenes
Thioketenes can be stabilized by either steric protection or by electronic effects. Thus, di-tert-butylthioketene is easily isolated and air-stable.[4] Several examples have been characterized by X-ray crystallography. The C=S distance is 157 pm and the C=C distance is 124 pm, both bonds being suitable for the C=C=S assignment. The violet color characteristic of thioketenes indicates the small HOMO-LUMO gap.[5] These compound are prepared by treatment of the acid chloride with phosphorus pentasulfide as described by the following idealized equation:
- RCH2COCl + P4S10 → RCH=C=S + HCl + "P4S9O"
Bis(trifluoromethyl)thioketene ((CF3)2C=C=S) is an example of an electronically stabilized thioketene.[6]
Reactions
Thioketenes are electrophilic. They add amines to give thioamides:[4]
- R2C=C=S + HNR'2 → R2CH−C(S)−NR'2
With peroxyacids, they produce thioketene-S-oxides:[5]
- R2C=C=S + R'CO3H → R2CH=C=S=O + R'CO2H
Thioketenes bind to metal carbonyls giving adducts.[7]
Related compounds
- carbon subsulfide (S=C=C=C=S).
References
- Noslash;rkjær, Kim; Senning, Aexander (1992). "Thio-, Seleno-, and Telluroketenes". Sulfur Reports. 11 (2): 361–384. doi:10.1080/01961779208046190.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Otto-Albrecht Neuman (Editor). Rompps Encyclopedia of Chemistry, Frank'sche Publishing House, Stuttgart, 1983, 8. Edition, p. 4242, ISBN 3-440-04513-7.
- Dekant, Wolfgang; Urban, Gudrun; Goersmann, Claus; Anders, M.W. (1991). "Thioketene formation from α-haloalkenyl 2-nitrophenyl disulfides: models for biological reactive intermediates of cytotoxic S-conjugates". J. Am. Chem. Soc. 113 (13): 5120–5122. doi:10.1021/ja00013a090.
- Elam, E. U.; Rash, F. H.; Dougherty, J. T.; Goodlett, V. W.; Brannock, K. C. (1968). "Di-tert-Butylthioketene". The Journal of Organic Chemistry. 33 (7): 2738–2741. doi:10.1021/jo01271a027.
- Schaumann, Ernst; Harto, Surya; Adiwidjaja, Gunadi (1979). "Kristall‐ und Molekülstruktur eines Ketens, eines Thioketens und eines Thioketen‐S‐oxids". Chemische Berichte. 112 (7): 2698–2708. doi:10.1002/cber.19791120738.
- Raasch, Maynard S. (1970). "Bis(trifluoromethyl)thioketene. I. Synthesis and Cycloaddition Reactions". J. Org. Chem. 35 (10): 3470–3483. doi:10.1021/jo00835a064.
- Seitz, Karsten; Benecke, Jörg; Behrens, Ulrich (1989). "Übergangsmetall-heteroallen-komplexe". Journal of Organometallic Chemistry. 371 (2): 247–256. doi:10.1016/0022-328x(89)88030-1.