Thiuram disulfide
Thiuram disulfides are a class of organosulfur compounds with the formula (R2NCSS)2. Many examples are known, but popular ones include R = Me and R = Et. They are disulfides obtained by oxidation of the dithiocarbamates. These compounds are used in sulfur vulcanization of rubber as well as in the manufacture of pesticides and drugs. They are typically white or pale yellow solids that are soluble in organic solvents.[1]
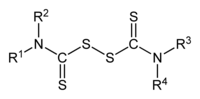
Preparation, structure, reactions
Thiuram disulfides are prepared by oxidizing the salts of the corresponding dithiocarbamates (e.g. sodium diethyldithiocarbamate). Typical oxidants employed include chlorine and hydrogen peroxide:
- 2 R2NCSSNa + Cl2 → (R2NCSS)2 + 2 NaCl
Thiuram disulfides react with Grignard reagents to give esters of dithiocarbamic acid, as in the preparation of methyl dimethyldithiocarbamate:[2]
- [Me2NC(S)S]2 + MeMgX → Me2NC(S)SMe + Me2NCS2MgX
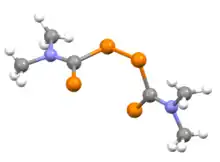
The compounds feature planar dithiocarbamate subunits and are linked by an S−S bond of 2.00 Å. The C(S)−N bond is short (1.33 Å), indicative of multiple bonding. The dihedral angle between the two dithiocarbamate subunits approaches 90°.[3]
Thiuram disulfides are weak oxidants. They can be reduced to dithiocarbamates. Treatment of a thiuram disulfide with triphenylphosphine, or with cyanide salts, yields the corresponding thiuram sulfide:
- (R2NCSS)2 + PPh3 → (R2NCS)2S + SPPh3
Chlorination of thiuram disulfide affords the thiocarbamoyl chloride.[4]
Applications
The tetramethyl derivative, known as thiram, is a widely used fungicide. The tetraethyl derivative, known as disulfiram, is commonly used to treat chronic alcoholism. It produces an acute sensitivity to alcohol ingestion by blocking metabolism of acetaldehyde by acetaldehyde dehydrogenase, leading to a higher concentration of the aldehyde in the blood, which in turn produces symptoms of a severe hangover.
Safety
In 2005–06, thiuram mix was the 13th most prevalent allergen in patch tests (3.9%).[5]
References
- Schubart, Rüdiger (2000). "Dithiocarbamic Acid and Derivatives". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a09_001.
- John R. Grunwell (1970). "Reaction of Grignard Reagents with Tetramethylthiuram Disulfide [yielding dithiocarbamates]". J. Org. Chem. 35 (5): 1500–1501. doi:10.1021/jo00830a052.
- Wang, Yu; Liao, J.-H. (1989). "Deformation Density Studies of Tetramethylthiuram Disulfide and Tetraethylthiuram Disulfide". Acta Crystallographica B. 45: 65. doi:10.1107/S0108768188010365.
- Goshorn, R. H.; Levis, Jr., W. W.; Jaul, E.; Ritter, E. J. (1955). "Diethylthiocarbamyl Chloride". Organic Syntheses. 35: 55. doi:10.15227/orgsyn.035.0055.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Zug, K. A.; Warshaw, E. M.; Fowler, J. F., Jr; Maibach, H. I.; Belsito, D. L.; Pratt, M. D.; Sasseville, D.; Storrs, F. J.; Taylor, J. S.; Mathias, C. G.; Deleo, V. A.; Rietschel, R. L.; Marks, J. (2009). "Patch-test results of the North American Contact Dermatitis Group 2005–2006". Dermatitis. 20 (3): 149–160. doi:10.2310/6620.2009.08097. PMID 19470301. S2CID 24088485.
{{cite journal}}: CS1 maint: multiple names: authors list (link)