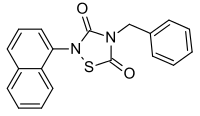
Tideglusib
Tideglusib (NP-12, NP031112) is a potent and irreversible[1] small molecule glycogen synthase kinase 3 (GSK-3) inhibitor.
 | |
 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C19H14N2O2S |
| Molar mass | 334.39 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Other GSK inhibitors
There are few classes of GSK-inhibitors, including lithium (Martinez et al., 2011), the small peptide L803mts10, and members of the thiazolidinedione family, containing inhibitors of GSK-3, such as TDZD-8 (Shapira et al., 2007) or Tideglusib® (Noscira, Madrid, and Spain), the latter having an irreversible inhibitory effect on GSK-3 (Dominguez et al., 2012). The inhibition of the GSK-3 pathways through distinct mechanisms has been associated with a wide range of adverse reactions, ranging from mild, such as vertigo—or diarrhea (del Ser et al., 2013)—to very severe, such as hypoglycemia—or tumorigenesis (Martinez et al., 2011). The use of Tideglusib specifically was associated with mild-moderate adverse reactions, which included transient increases in serum creatine kinase, ALT—or gGT—diarrhea, nausea, cough, fatigue, and headache (del Ser et al., 2013). In a phase-IIa clinical trial for Alzheimer's disease, the treatment was discontinued, due to lack of efficacy (del Ser et al., 2013).
Potential applications
Tideglusib is or has been under investigation for multiple applications:
- Alzheimer's disease and progressive supranuclear palsy. Both clinical trials were discontinued in 2011 (PSP) and 2012 (Alzheimer's disease) due to lack of efficacy[2] [3][4][5][6]
- Tooth repair mechanisms that promotes dentine reinforcement of a sponge structure until the sponge biodegrades, leaving a solid dentine structure. In 2016, the results of animal studies were reported in which 0.14 mm holes in mouse teeth were permanently filled.[7][8]
- Tideglusib is being studied in Phase II clinical trials as a treatment for congenital/juvenile-onset myotonic muscular dystrophy type I.[9]
References
- Domínguez JM, Fuertes A, Orozco L, del Monte-Millán M, Delgado E, Medina M (January 2012). "Evidence for irreversible inhibition of glycogen synthase kinase-3β by tideglusib". The Journal of Biological Chemistry. 287 (2): 893–904. doi:10.1074/jbc.M111.306472. PMC 3256883. PMID 22102280.
- Del Ser T (2010). "Phase IIa clinical trial on Alzheimer's disease with NP12, a GSK3 inhibitor". Alzheimer's & Dementia. 6 (4): S147. doi:10.1016/j.jalz.2010.05.455. S2CID 54293332.
- Eldar-Finkelman H, Martinez A (2011). "GSK-3 Inhibitors: Preclinical and Clinical Focus on CNS". Frontiers in Molecular Neuroscience. 4: 32. doi:10.3389/fnmol.2011.00032. PMC 3204427. PMID 22065134.
- del Ser T, Steinwachs KC, Gertz HJ, Andrés MV, Gómez-Carrillo B, Medina M, et al. (2013). "Treatment of Alzheimer's disease with the GSK-3 inhibitor tideglusib: a pilot study". Journal of Alzheimer's Disease. 33 (1): 205–15. doi:10.3233/JAD-2012-120805. PMID 22936007. S2CID 21892732.
- "FDA Grants Fast Track Status to Tideglusib (ZentylorTM) for Progressive Supranuclear Palsy". PR Newswire Europe Including UK Disclose. 10 September 2010. ProQuest 750175748.
- Domínguez JM, Fuertes A, Orozco L, del Monte-Millán M, Delgado E, Medina M (January 2012). "Evidence for irreversible inhibition of glycogen synthase kinase-3β by tideglusib". The Journal of Biological Chemistry. 287 (2): 893–904. doi:10.1074/jbc.M111.306472. PMC 3256883. PMID 22102280.
- Neves VC, Babb R, Chandrasekaran D, Sharpe PT (January 2017). "Promotion of natural tooth repair by small molecule GSK3 antagonists". Scientific Reports. 7: 39654. Bibcode:2017NatSR...739654N. doi:10.1038/srep39654. PMC 5220443. PMID 28067250.
- Gallagher J (2017-01-09). "'Tooth repair drug' may replace fillings". BBC News. Retrieved 2017-01-09.
- "AMO-2". AMO Pharmaceuticals. Retrieved 2017-09-21.