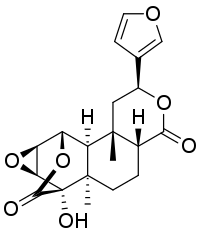
Tinosporide
Tinosporide is a chemical compound classified as a diterpenoid and a furanolactone. It was first isolated from the plant Tinospora cordifolia, from which it derives its name.[1][2] It has since been found in other plants of the genus Tinospora, such as Tinospora glabra.
 | |
| Names | |
|---|---|
| IUPAC name
(2S,4aR,6aR,7S,7aS,8aS,9S,9aS,9bS)-2-(3-Furanyl)dodecahydro-7-hydroxy-6a,9b-dimethyl-9,7-(epoxymethano)-4H-oxireno[6,7]naphtho[2,1-c]pyran-4,11-dione | |
| Other names
2,3-Epoxycolumbin; Jateorin; 5-(furan-3-yl)-12-hydroxy-3,11-dimethyl-6,14,16-trioxapentacyclo[10.3.2.02,11.03,8.013,15]heptadecane-7,17-dione | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| |
| |
| Properties | |
| C20H22O7 | |
| Molar mass | 374.389 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Because Tinospora cordifolia has been used in traditional herbal medicine, there has been research directed at exploring the potential pharmacology of tinosporide and related compounds.[3]
Related compounds
Other diterpenoid furanolactones with a similar structure include columbin, palmarin, and chasmanthin.
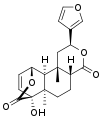 Columbin
Columbin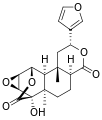 Palmarin
Palmarin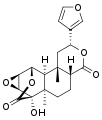 Chasmanthin
Chasmanthin
External links
- Swaminathan, K.; Sinha, U. C.; Bhatt, R. K.; Sabata, B. K.; Tavale, S. S. (1989). "Structure of tinosporide, a diterpenoid furanolactone from Tinospora cordifolia Miers". Acta Crystallographica Section C. 45 ( Pt 1): 134–136. doi:10.1107/s0108270188009953. PMID 2610955.
- Sharma, Priyanka; Dwivedee, Bharat P.; Bisht, Dheeraj; Dash, Ashutosh K.; Kumar, Deepak (2019). "The chemical constituents and diverse pharmacological importance of Tinospora cordifolia". Heliyon. 5 (9): e02437. Bibcode:2019Heliy...502437S. doi:10.1016/j.heliyon.2019.e02437. PMC 6827274. PMID 31701036.
- Pathak, Ashish K.; Jain, Dharam C.; Sharma, Ram P. (1995). "Chemistry and Biological Activities of the Genera Tinospora". International Journal of Pharmacognosy. 33 (4): 277–287. doi:10.3109/13880209509065379.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.