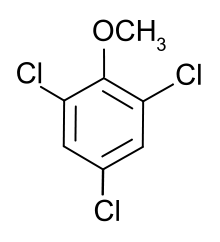
2,4,6-Trichloroanisole
2,4,6-Trichloroanisole (TCA) is a chemical compound that represents one of the strongest of off-flavors, substances "generated naturally in foods/beverages [that considerably] deteriorate the quality" of such products.[1] As of 2000, TCA was considered the primary chemical compound responsible for the phenomenon of cork taint in wines,[2][1] and it has an unpleasant earthy, musty and moldy smell.
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
1,3,5-Trichloro-2-methoxybenzene | |
| Other names
2,4,6-Trichloroanisole TCA 2,4,6-Trichloromethoxybenzene | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.001.585 |
| EC Number |
|
| KEGG | |
PubChem CID |
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C7H5Cl3O | |
| Molar mass | 211.47 g·mol−1 |
| Melting point | 60 to 62 °C (140 to 144 °F; 333 to 335 K) |
| Boiling point | 140 °C (284 °F; 413 K) at 28 Torr |
| Hazards | |
| GHS labelling: | |
 | |
| Warning | |
| H302, H319, H413 | |
| P264, P270, P273, P280, P301+P312, P305+P351+P338, P330, P337+P313, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Chemically, TCA is a chlorinated derivative of anisole, and it is a fungal metabolite of 2,4,6-trichlorophenol.[3] More generally, it may be produced when naturally occurring airborne fungi and bacteria are presented with chlorinated phenolic compounds, which they then convert into chlorinated anisole derivatives. Species implicated include those of the genera Aspergillus, Penicillium, Actinomycetes, Botrytis (e.g. Botrytis cinerea), Rhizobium, or Streptomyces.[4]
The chlorophenol precursor, 2,4,6-trichlorophenol, is used as a fungicide; more generally, related compounds can originate as contaminants found in some pesticides and wood preservatives, or as by-products of the chlorine bleaching process used to sterilize or bleach wood, paper, and other materials. The precursors can result from the reaction of hypochlorites with lignin, or can also migrate from other manufactured objects (e.g., shipping pallets treated by chlorophenols or materials stored in the presence of trichlorophenol-treated fiberboard).
TCA has also been suggested as cause of the "Rio defect" in coffees from Brazil and other parts of the world,[5] which refers to a taste described as "medicinal, phenolic, or iodine-like".[3] In investigation of the mechanism of its role in producing off-flavor effects, it was found to "attenuate olfactory transduction by suppressing cyclic nucleotide-gated channels, without evoking odorant responses."[1]
Further reading
- Marsili, R. (2000). "Solid-Phase Microextraction: Food Technology Applications". In Wilson, Ian D. (ed.). Encyclopedia of Separation Science. New Yor, NY: Academic Press. pp. 4178–4190. doi:10.1016/B0-12-226770-2/06791-0. ISBN 9780122267703.
Over the last two decades, the incidence of mouldy and musty off-flavours in cork-sealed wines has increased significantly. 2,4,6-Trichloroanisole (TCA) has been identified as the primary chemical responsible for cork taint. The human olfactometry threshold for TCA is 4–10 ng L−1 in white wine and 50 ng L−1 in red wine. In the case of wine, a worldwide loss of roughly US$1 billion per year is attributed to cork taint.
- Science Direct Staff (June 2023). "2-4-6-Trichloroanisole" (Science Direct citation sample/listing). Retrieved June 26, 2023.
- Buser, H.R.; Zanier, C. & Tanner, H. (1982). "Identification of 2,4,6-Trichloroanisole as a Potent Compound Causing Cork Taint in Wine". Journal of Agricultural and Food Chemistry. 30 (2): 359–362. doi:10.1021/jf00110a037.
{{cite journal}}: CS1 maint: multiple names: authors list (link) An early primary research report on the role of TCA in cork taint.
See also
References
- Takeuchi, Hiroko; Kato, Hiroyuki & Kurahashi, Takashi (2013-09-16). "2,4,6-Trichloroanisole is a Potent Suppressor of Olfactory Signal Transduction". Proceedings of the National Academy of Sciences. 110 (40): 16235–16240. Bibcode:2013PNAS..11016235T. doi:10.1073/pnas.1300764110. ISSN 1091-6490. PMC 3791788. PMID 24043819.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Marsili, R. (2000). "Solid-Phase Microextraction: Food Technology Applications". In Wilson, Ian D. (ed.). Encyclopedia of Separation Science. New Yor, NY: Academic Press. pp. 4178–4190. doi:10.1016/B0-12-226770-2/06791-0. ISBN 9780122267703.
Over the last two decades, the incidence of mouldy and musty off-flavours in cork-sealed wines has increased significantly. 2,4,6-Trichloroanisole (TCA) has been identified as the primary chemical responsible for cork taint. The human olfactometry threshold for TCA is 4–10 ng L−1 in white wine and 50 ng L−1 in red wine. In the case of wine, a worldwide loss of roughly US$1 billion per year is attributed to cork taint.
- Spadone, Jean Claude; Takeoka, Gary & Liardon, Remy (1990). "Analytical Investigation of Rio Off-Flavor in Green Coffee". Journal of Agricultural and Food Chemistry. 38: 226–233. doi:10.1021/jf00091a050.
{{cite journal}}: CS1 maint: multiple names: authors list (link) Note, at best, this source states that 2,4,6-trichlorophenol is "the probable precursor of TCA". - With regard to circumstantial evidence, Spodone, et al., op. cit., note that Rio off-flavor is associated with "beans heavily infested with various fungi (Aspergilli, Fusaria, Penicillia, Rhizopus, etc.) and bacteria (Lactobacilli, Streptrococci)".
- These include Central and South America.