Trichothecium roseum
Trichothecium roseum is a fungus in the division Ascomycota first reported in 1809.[1] It is characterized by its flat and granular colonies which are initially white and develop to be light pink in color.[1] This fungus reproduces asexually through the formation of conidia with no known sexual state.[1] Trichothecium roseum is distinctive from other species of the genus Trichothecium in its characteristic zigzag patterned chained conidia.[2] It is found in various countries worldwide and can grow in a variety of habitats ranging from leaf litter to fruit crops.[2] Trichothecium roseum produces a wide variety of secondary metabolites including mycotoxins, such as roseotoxins and trichothecenes, which can infect and spoil a variety of fruit crops.[1] It can act as both a secondary and opportunistic pathogen by causing pink rot on various fruits and vegetables and thus has an economical impact on the farming industry.[1] Secondary metabolites of T. roseum, specifically Trichothecinol A, are being investigated as potential anti-metastatic drugs. Several agents including harpin, silicon oxide, and sodium silicate are potential inhibitors of T. roseum growth on fruit crops.[3][4][5] Trichothecium roseum is mainly a plant pathogen and has yet to show a significant impact on human health.[1]
| Trichothecium roseum | |
|---|---|
 | |
| Scientific classification | |
| Domain: | Eukaryota |
| Kingdom: | Fungi |
| Division: | Ascomycota |
| Class: | Sordariomycetes |
| Order: | Hypocreales |
| Genus: | Trichothecium |
| Species: | T. roseum |
| Binomial name | |
| Trichothecium roseum (Pers.) Link (1809) | |
| Synonyms | |
| |
History and classification
The genus Trichothecium is small and heterogeneous comprising seventy-three recorded species.[1] This genus was first reported in 1809.[1] The main members of the genus include Trichothecium polybrochum, Trichothecium cystosporium, Trichothecium pravicovi, and Trichothecium roseum.[1] Trichothecium roseum has morphologically different conidiophores and conidia than the other three main species, which made development of these features the center of extensive study throughout the years.[1] Since Trichothecium fungi lack a sexual phase, systematic classification was not uniform following their discovery.[1] These fungi were initially grouped into Fungi imperfecti under the form classification Deuteromycetes.[1] In 1958, Tubaki expanded Hughes' classification of soil Hyphomycetes, part of the form class of Fungi imperfecti, by adding a ninth section in order to accommodate T. roseum and its unique conidial apparatus.[6][7] Trichothecium has now been classified under the class Sordariomycetes, phylum Ascomycota.[1]
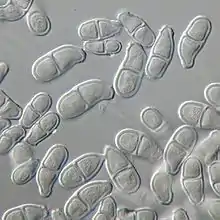
Morphology
Trichothecium roseum colonies are flat, granular, and powdery in appearance.[1][2] The color of the colonies appears to be white initially and develop into a light pink to peach color.[1] The genus Trichothecium is characterized by its pinkish colored colonies.[8]
Conidiophores of T. roseum are usually erect and are 200-300μm in length.[9] They arise singly or in loose groups.[1] Conidiophores are simple hyphae,[10] which are septate in their lower half,[6] and bear clusters of conidia at the tip.[2] These conidiophores are indistinguishable from vegetative hyphae until production of the first conidium.[1] Conidium development is distinctive[2] and was first described by Ingold in 1956.[6] Conidia arise as blowouts from the side of the conidiophore apex which is thus incorporated into the base of each spore.[6] After the first conidium is blown out, before it matures, the apex of the conidiophore directly below blows out a second conidium from the opposite side.[6] Conidia are pinched out from the conidiophore one after another in alternating directions in order to form the characteristic zigzag patterned chain.[1] Conidia of T.roseum (15-20 × 7.5-10 μm)[9] are smooth and clavate.[1] Each conidium is two celled with the apical cell being larger than the curved basal cell.[1] Conidia are light pink and appear translucent under the microscope.[1] They appear a more saturated pink colour when grown in masses in culture or on the host surface.[1]
Growth and physiology
Trichothecium roseum reproduces asexually by the formation of conidia with no known sexual stage.[1] Trichothecium roseum is relatively fast-growing as it can form colonies reaching 9 cm (4 in) in diameter in ten days at 20 °C (68 °F) on malt extract agar.[8] This fungus grows optimally at 25 °C (77 °F) with a minimum and maximum growing temperature of 15 °C (59 °F) and 35 °C (95 °F) respectively.[8] Trichothecium roseum can tolerate a wide pH range but grows optimally at a pH of 6.0. Sporulation occurs rapidly at pH 4.0-6.5 and a combination of low temperature (15 °C (59 °F)) and high glucose concentration can increase the size of conidia.[8] Treatment of T. roseum with colchicine increases the number of nuclei in conidia, growth rate, and biosynthetic activities.[8] There are a variety of sugars that T. roseum can utilize including D-fructose, sucrose, maltose, lactose, raffinose, D-galactose, D-glucose, arabinose, and D-mannitol.[8] Good growth also occurs in the presence of various amino acids including L-methionine, L-isoleucine, L-tryptophan, L-alanine, L-norvaline, and L-norleucine.[8]
Secondary metabolites
Trichothecium roseum can produce numerous secondary metabolites that include toxins, antibiotics, and other biologically active compounds.[1] Diterpenoids produced include rosolactone, rosolactone acetate, rosenonolactone, desoxyrosenonolactone, hydroxyrosenonolactones, and acetoxy-rosenonolactone. Several sesquiterpenoids are also produced by T. roseum including crotocin, trichothecolone, trichothecin, trichodiol A, trichothecinol A/B/C, trichodiene, and roseotoxin.[1][8][11]
Biomedical applications
Trichothecium roseum was found to antagonize pathogenic fungi, such as Pyricularia oryzae (Magnaporthe oryzae) and Phytophthora infestans, in vitro.[12] It was suggested that the antifungal compound trichothecin was the main contributor to this action.[12] In other studies trichothecinol B isolated from T. roseum displayed modest antifungal activity against Cryptococcus albidus and Saccharomyces cerevisiae.[13]
Various studies have indicated that Trichothecinol A isolated from T. roseum strongly inhibited TPA-induced tumour promotion on mouse skin in carcinogenesis tests and therefore may be valuable for further investigation as cancer preventive agent.[13][14][15] Anti-cancer studies have also shown that Trichothecinol A significantly inhibits cancer cell migration and therefore can be developed as a potential new anti-metastatic drug.[15]
Habitat and ecology
Trichothecium roseum is a saprophyte[10] and is found worldwide.[8] It has been found in soils in various countries including Poland, Denmark, France, Russia, Turkey, Israel, Egypt, the Sahara, Chad, Zaïre, central Africa, Australia, Polynesia, India, China, and Panama.[8] Known habitats of T. roseum include uncultivated soils, forest nurseries, forest soils under beech trees, teak, cultivated soils with legumes, citrus plantations, heathland, dunes, salt-marshes, and garden compost.[8] Commonly, this fungus can be isolated from the tree leaf litter of various trees including birch, pine, fir, cotton, and palm.[8] It has also been isolated from several food sources such as barley, wheat, oats, maize, apples, grapes, meat products, cheese, beans, hazelnuts, pecans, pistachios, peanuts, and coffee.[10] Levels of T. roseum in foods other than fruits are generally low.[10]
Plant pathology
There are approximately two hundred twenty-two different plant hosts of T. roseum found worldwide.[1] Trichothecium roseum causes pink rot on various fruits and vegetables.[1] It is considered both a secondary and opportunistic pathogen since it tends to enter the fruit/vegetable host through lesions that were caused by a primary pathogen.[1] Disease caused by this fungus is characterized by the development of white powdery mold that eventually turns pink.[1] Antagonistic behaviours of T. roseum with certain plant pathogenic fungi was reported by Koch in 1934.[16] He started that T. roseum actively parasitized stroma of Dibotryon morbosum which causes black knot disease in cherry, plum, and apricot trees.[16]
Apple disease
Trichothecium roseum is known to produce pink rot on apples particularly following an apple scab infection caused by Venturia inaequalis.[1] Studies have shown that roseotoxin B, a secondary metabolite of T. roseum, can penetrate apple peels and cause lesions.[17] Trichothecium roseum also causes apple core rot which is a serious problem in China.[18] Core rot not only causes economic loss but it is also associated with high levels of mycotoxin production.[18] There have been reports of the presence of trichothecenes, specifically T-2 toxin, in infected apples in China.[18] T-2 toxin has the highest toxicity of the trichothecenes and poses a threat to individuals who consume these infected apples due to its carcinogenicity, neurotoxicity, and immunotoxicity.[18]
Grape disease
Trichothecium roseum was identified, along with Acremonium acutatum, as the two strains of pathogenic fungi which caused white stains on harvested grapes in Korea.[19] The presence of mycelia on the surface of the grapes resulted in a white stained, powdery mildew appearance.[19] Trichothecium roseum was identified using fungal morphology and nucleotide sequencing by PCR.[19] It appears as though the fungus covers the surface of the grape only and does not penetrate into the tissue.[19] This stain lowers the quality of the grapes and causes serious economic losses.[19]
Trichothecin, trichothecolone, and rosenonolactone, which are secondary metabolites of T. roseum, were detected in wines.[20] Presence of small quantities of trichothecin can inhibit alcohol fermentation.[20] Trichothecium roseum rot has been reported to be increasing in wineries in Portugal.[20] In this case, T. roseum appeared to grow over rotten grapes that were infected with gray rot.[20] Mycotoxins were only detected in wines that were made with grapes that had gray rot and thus these toxins may be indicators of poor quality grapes.[20] Grape contamination by T. roseum appears to be prominent in temperate climates.[20]
Other fruit disease
Cases of T. roseum pink rot have been reported on numerous other fruits, however detailed studies have yet to be pursued.[1] Pink T. roseum rot has been reported on tomatoes in Korea and Pakistan.[21][22] It also causes pink rot in muskmelons and watermelons in Japan, the United States, South America, India, and the United Kingdom.[1] Trichothecium roseum is reported to grow also on bananas and peaches.[1]
Prevention of plant disease
Preventative measures can be taken to avoid growth of T. roseum in fruit crops.[23] These include ensuring adequate ventilation in the storage facility, avoiding injuring and bruising the fruit, and ensuring adequate storage temperatures.[23] Pre- and postharvest applications have been suggested as measures to control T. roseum production on fruit crops.[1] In particular, studies have been done on testing various compounds to prevent T. roseum growth on several melon types.[3][4][5] Harpin was inoculated on harvested Hami melons and caused significantly reduced lesion diameter and thus decreased T. roseum growth.[3] Silicon oxide and sodium silicate also reduced the severity of pink rot and lesion diameter in harvested Hami melons.[4] Pre-harvest inoculation of harpin on muskmelons decreased the amount of pink rot caused by T. roseum on harvested melons.[5]
References
- Batt, C.A.; Tortorello, M (2014). Encyclopedia of food microbiology (2 ed.). London: Elsevier Ltd. p. 1014. ISBN 978-0-12-384730-0.
- Onions, A.H.S.; Allsopp, D.; Eggins, H.O.W. (1981). Smith's introduction to industrial mycology (7th ed.). London, UK: Arnold. ISBN 978-0-7131-2811-6.
- Yang, B; Shiping, T; Jie, Z; Yonghong, G (2005). "Harpin induces local and systemic resistance against Trichothecium roseum in harvested Hami melons". Postharvest Biology and Technology. 38 (2): 183–187. doi:10.1016/j.postharvbio.2005.05.012.
- Guo, Y; Liu, L; Zhao, J; Bi, Y (2007). "Use of silicon oxide and sodium silicate for controlling Trichothecium roseum postharvest rot in Chinese cantaloupe (Cucumis melo L.)". International Journal of Food Science & Technology. 42 (8): 1012–1018. doi:10.1111/j.1365-2621.2006.01464.x.
- Wang, J; Bi, Y; Wang, Y; Deng, J; Zhang, H; Zhang, Z (2013). "Multiple preharvest treatments with harpin reduce postharvest disease and maintain quality in muskmelon fruit (cv. Huanghemi)". Phytoparasitica. 42 (2): 155–163. doi:10.1007/s12600-013-0351-8. S2CID 6795039.
- Barron, George L. (1968). The genera of Hyphomycetes from soil. Baltimore, MD: Williams & Wilkins. ISBN 9780882750040.
- Kendrick, W. B.; Cole, G. T. (1969). "Conidium ontogeny in hyphomycetes and its meristem arthrospores". Canadian Journal of Botany. 47 (2): 345–350. doi:10.1139/b69-047.
- Domsch, K.H.; Gams, Walter; Andersen, Traute-Heidi (1980). Compendium of soil fungi (2nd ed.). London, UK: Academic Press. ISBN 9780122204029.
- Watanabe, Tsuneo (2009). Pictorial atlas of soil and seed fungi : morphologies of cultured fungi and key to species (3rd ed.). Boca Raton, Fla.: CRC. ISBN 978-1-4398-0419-3.
- Pitt, J.I.; Hocking, A.D. (1999). Fungi and food spoilage (2nd ed.). Gaithersburg, Md.: Aspen Publications. ISBN 978-0834213067.
- Cole, Richard; Jarvis, Bruce; Schweikert, Milbra (2003). Handbook of secondary fungal metabolites. Oxford: Academic. ISBN 978-0-12-179460-6.
- Zhang, XiaoMei; Li, GuoHong; Ma, Juan; Zeng, Ying; Ma, WeiGuang; Zhao, PeiJi (9 January 2011). "Endophytic fungus Trichothecium roseum LZ93 antagonizing pathogenic fungi in vitro and its secondary metabolites". The Journal of Microbiology. 48 (6): 784–790. doi:10.1007/s12275-010-0173-z. PMID 21221935. S2CID 11928442.
- Konishi, Kazuhide; Iida, Akira; Kaneko, Masafumi; Tomioka, Kiyoshi; Tokuda, Harukuni; Nishino, Hoyoku; Kumeda, Yuko (June 2003). "Cancer preventive potential of trichothecenes from Trichothecium roseum". Bioorganic & Medicinal Chemistry. 11 (12): 2511–2518. doi:10.1016/S0968-0896(03)00215-3. PMID 12757719.
- Iida, Akira; Konishi, Kazuhide; Kubo, Hiroki; Tomioka, Kiyoshi; Tokuda, Harukuni; Nishino, Hoyoku (December 1996). "Trichothecinols A, B and C, potent anti-tumor promoting sesquiterpenoids from the fungus Trichothecium roseum". Tetrahedron Letters. 37 (51): 9219–9220. doi:10.1016/S0040-4039(96)02187-9.
- Taware, R; Abnave, P; Patil, D; Rajamohananan, P; Raja, R; Soundararajan, G; Kundu, G; Ahmad, A (2014). "Isolation, purification and characterization of Trichothecinol-A produced by endophytic fungus Trichothecium sp. and its antifungal, anticancer and antimetastatic activities". Sustainable Chemical Processes. 2 (1): 8. doi:10.1186/2043-7129-2-8.
- Freeman, G.G.; Morrison, R.I. (1949). "Metabolic products of Trichothecium roseum Link". Biochemical Journal. 45 (2): 191–199. doi:10.1042/bj0450191. PMC 1274970. PMID 16748611.
- Žabka, Martin; Drastichová, Kamila; Jegorov, Alexandr; Soukupová, Julie; Nedbal, Ladislav (July 2006). "Direct Evidence of Plant-pathogenic Activity of Fungal Metabolites of Trichothecium roseum on Apple". Mycopathologia. 162 (1): 65–68. doi:10.1007/s11046-006-0030-0. PMID 16830194. S2CID 23611080.
- Tang, Y; Xue, H; Bi, Y; Li, Y; Wang, Y; Zhao, Y; Shen, K (2014). "A method of analysis for T-2 toxin and neosolaniol by UPLC-MS/MS in apple fruit inoculated with Trichothecium roseum". Food Additives & Contaminants: Part A. 32 (4): 480–487. doi:10.1080/19440049.2014.968884. PMID 25254921. S2CID 1277261.
- Oh, S.Y.; Nam, K.W.; Yoon, D.H. (2014). "Identification of and isolated from Grape with White Stain Symptom in Korea". Mycobiology. 42 (3): 269–273. doi:10.5941/MYCO.2014.42.3.269. PMC 4206793. PMID 25346604.
- Serra, R; Braga, A; Venâncio, A (2005). "Mycotoxin-producing and other fungi isolated from grapes for wine production, with particular emphasis on ochratoxin A". Research in Microbiology. 156 (4): 515–521. doi:10.1016/j.resmic.2004.12.005. hdl:1822/2614. PMID 15862450.
- Han, K.S.; Lee, S.C.; Lee, J.S.; Soh, J.W. (2012). "First Report of Pink Mold Rot on Tomato Fruit Caused by Trichothecium roseum in Korea". Research in Plant Disease. 18 (4): 396–398. doi:10.5423/RPD.2012.18.4.396.
- Hamid, M.I.; Hussain, M; Ghazanfar, M.U.; Raza, M; Liu, X.Z. (2014). "Causes Fruit Rot of Tomato, Orange, and Apple in Pakistan". Plant Disease. 98 (9): 1271. doi:10.1094/PDIS-01-14-0051-PDN. PMID 30699663.
- Rees, D; Farrell, G; Orchard, J.E. (2006). Crop post-harvest : science and technology. Oxford: Blackwell Science. p. 464. ISBN 978-0-632-05725-2.
External links
- Trichothecium roseum in MycoBank.
- Trichothecium roseum in Index Fungorum
 Data related to Trichothecium roseum at Wikispecies
Data related to Trichothecium roseum at Wikispecies Media related to Trichothecium roseum at Wikimedia Commons
Media related to Trichothecium roseum at Wikimedia Commons