Trifluoroacetonitrile
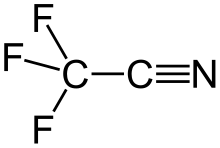
Trifluoroacetonitrile is a nitrile with the chemical formula CF3CN.
 | |
| Identifiers | |
|---|---|
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.005.948 |
| EC Number |
|
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C2F3N | |
| Molar mass | 95.024 g·mol−1 |
| Appearance | colourless gas[1] |
| Boiling point | −64 °C (1013 hPa)[1] |
| insoluble[1] | |
| Related compounds | |
Related compounds |
acetonitrile trifluoromethylisocyanide trichloroacetonitrile |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Production
Trifluoroacetonitrile can be produced by dehydration of trifluoroacetamide with trifluoroacetic anhydride in pyridine or carbon tetrachloride.[2][3] This synthesis route was first described by Frédéric Swarts in 1922.[4]
Trifluoroacetonitrile can also be produced by reacting 1,1,1-trichloro-2,2,2-trifluoroethane and ammonia at 610 °C.[5]
Properties
Trifluoroacetonitrile is a colourless gas that is insoluble in water.[1] Solid trifluoroacetonitrile's crystal structure is orthorhombic.[6]
Uses
Trifluoroacetonitrile can be used to prepare other chemicals such as 3-(trifluoromethyl)isoquinoline and 2,4-bis(trifluoromethyl)pyrimidine.[7][8]
References
- Sigma-Aldrich Co., product no. 544078.
- Marshall H. Parker (2004), "A Convenient Preparation of Trifluoroacetonitrile: Application to the Synthesis of a Novel Pyrimidinone Building Block", Synthetic Communications (in German), vol. 34, no. 5, pp. 903–907, doi:10.1081/SCC-120028363, S2CID 97226252
- "Synthesis method for pesticide intermediate trifluoroacetonitrile". google.com.
- F. Swarts, Bulletin des Sociétés Chimiques Belges, 1922, Vol 31, S. 364–365.
- R. E. Banks, M. G. Barlow (2007), Fluorocarbon and Related Chemistry: Volume 1 (in German), Royal Society of Chemistry, ISBN 978-1-84755-601-1
- H. F. Shurvell, J. A. Faniran (1970), "The infrared and Raman spectra of solid trifluoroacetonitrile", Journal of Molecular Spectroscopy (in German), vol. 33, no. 3, pp. 436–447, Bibcode:1970JMoSp..33..436S, doi:10.1016/0022-2852(70)90137-2
- Valentine Nenajdenko (2014), Fluorine in Heterocyclic Chemistry Volume 2: 6-Membered Heterocycles (in German), Springer, ISBN 978-3-319-04435-4
- Klaus Burger, Ulrike Waßmuth, Friedrich Hein, Silvia Rottegger (1984), "Trifluormethyl-substituierte Pyrimidine aus Enaminen und Trifluoracetonitril", Liebigs Annalen der Chemie (in German), vol. 1984, no. 5, pp. 991–1002, doi:10.1002/jlac.198419840517
{{citation}}: CS1 maint: multiple names: authors list (link)
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.