Trithiazyl trichloride
Trithiazyl trichloride is the inorganic compound with the formula (NSCl)3. A white solid, it is a precursor to other sulfur nitrides,[1] but has no commercial applications.
 | |
 | |
| Names | |
|---|---|
| Other names
thionitrosyl chloride | |
| Identifiers | |
| |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| (NSCl)3 | |
| Molar mass | 244.55 g·mol−1 |
| Appearance | white solid |
| Melting point | 168 °C (334 °F; 441 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Structure
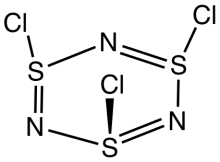
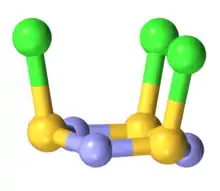
The molecule is a 6-membered ring of alternating nitrogen and sulfur atoms, where each sulfur atom is attached to one chlorine atom by a single bond. The molecule contains alternating single and double bonds in the S3N3 core. The molecule has C3v symmetry. The S3N3 core is slightly ruffled structure with S-N distances of 160.5 pm. The S-Cl distances are 208 pm, and the chlorine atoms are mutually cis. The S centers are tetravalent and pyramidal. In contrast to the NSCl connectivity, nitrosyl chloride has the connectivity ONCl.[2][3]
Synthesis and reactions
Trithiazyl trichloride is obtained by chlorination of tetrasulfur tetranitride:
- 3 S4N4 + 6 Cl2 → 4 (NSCl)3
At 100 °C in vacuum, thiazyl chloride trimer undergoes cracking to thiazyl chloride monomer, which is a green gas.
- (−N=S(−Cl)−)3 → 3 N≡S−Cl
In N≡S−Cl, chlorine is bonded to sulfur, in contrast to nitrosyl chloride O=N–Cl, where chlorine is bonded to nitrogen. In contrast, with six fewer electrons, cyanuric chloride is a planar ring.
Alkoxide or silver salts displace the chlorides:[4]
- (-NS(Cl)-)3 + 3 NaOR → (-NS(OR)-)3 + 3 NaCl
- (-NS(Cl)-)3 + 3 AgX → (-NS(X)-)3 + 3 AgCl
Treating thiazyl chloride with sulfur in the presence of antimony pentachloride gives dithionitronium hexachloroantimonate:[5]
- SNCl + S + SbCl5 → [NS2]SbCl6
It reacts with nitriles to dithiadiazolium chlorides:[2]
- 6 RCN + 4 (NSCl)3 → 6 [RCN2S2]+Cl− + 3 Cl2 + 3 N2
The compound oxidizes to the S(VI) compound (NSOCl)3, which exists as isomers.
References
- Jolly, William L.; Maguire, Keith D. (1967). "Sulfur Nitrogen Chlorides". Inorganic Syntheses. IX: 102. doi:10.1002/9780470132401.ch27. ISBN 978-0-470-13240-1.
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- Wiegers, G. A.; Vos, A. (1966). "The Crystal Structures of Two Sulfur-Nitrogen Compounds with (S-N)3 Rings. II. Trithiazylchloride, (NSCl)3, at -130 C". Acta Crystallographica. 20 (2): 192. doi:10.1107/s0365110x66000410.
- Rawson, Jeremy M.; Banister, Arthur J.; Lavender, Ian (1995). "The Chemistry of Dithiadiazolylium and Dithiadiazolyl Rings". Adv. Heterocyc. Chem. 62: 146–147. doi:10.1016/S0065-2725(08)60422-5.
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.