Tropodithietic acid
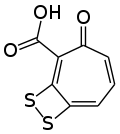
Tropodithietic acid is a tropolone derivative produced by the marine bacteria Phaeobacter piscinae, Phaeobacter inhibens and Phaeobacter gallaeciensis.[1][2] Its structure is composed by a dithiete moiety fused to tropone-2-carboxylic acid.
 | |
| Names | |
|---|---|
| Preferred IUPAC name
3-Oxo-8,9-dithiabicyclo[5.2.0]nona-1,4,6-triene-2-carboxylic acid | |
| Other names
TDA | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.233.118 |
| EC Number |
|
PubChem CID |
|
| |
| |
| Properties | |
| C8H4O3S2 | |
| Molar mass | 212.24 g·mol−1 |
| Hazards | |
| GHS labelling: | |
 | |
| Warning | |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
References
- Rabe, Patrick; Klapschinski, Tim A; Brock, Nelson L; Citron, Christian A; D’Alvise, Paul; Gram, Lone; Dickschat, Jeroen S (6 August 2014). "Synthesis and bioactivity of analogues of the marine antibiotic tropodithietic acid". Beilstein Journal of Organic Chemistry. 10: 1796–1801. doi:10.3762/bjoc.10.188. PMC 4142847. PMID 25161739.
- Beyersmann, Paul G.; Tomasch, Jürgen; Son, Kwangmin; Stocker, Roman; Göker, Markus; Wagner-Döbler, Irene; Simon, Meinhard; Brinkhoff, Thorsten (December 2017). "Dual function of tropodithietic acid as antibiotic and signaling molecule in global gene regulation of the probiotic bacterium Phaeobacter inhibens". Scientific Reports. 7 (1): 730. Bibcode:2017NatSR...7..730B. doi:10.1038/s41598-017-00784-7. PMC 5429656. PMID 28389641.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.