List of synthetic polymers
Some familiar household synthetic polymers include: Nylons in textiles and fabrics, Teflon in non-stick pans, Bakelite for electrical switches, polyvinyl chloride (PVC) in pipes, etc. The common PET bottles are made of a synthetic polymer, polyethylene terephthalate. The plastic kits and covers are mostly made of synthetic polymers like polythene, and tires are manufactured from polybutadienes.[1] However, due to the environmental issues created by these synthetic polymers which are mostly non-biodegradable and often synthesized from petroleum, alternatives like bioplastics are also being considered. They are however expensive when compared to the synthetic polymers.[2]
Artificial polymer: Man-made polymer that is not a biopolymer.
Note 1: Artificial polymer should also be used in the case of chemically
modified biopolymers.Note 2: Biochemists are now capable of synthesizing copies of biopolymers
that should be named Synthetic biopolymer to make a distinction
with true biopolymers.Note 3: Genetic engineering is now capable of generating non-natural analogues
of biopolymers that should be referred to as artificial biopolymers, e.g.,
artificial protein, artificial polynucleotide, etc.[3]
Inorganic polymers
- Polysiloxane
- Polyphosphazene
- Polyborazyline
Organic polymers
The eight most common types of synthetic organic polymers, which are commonly found in households are:
- Low-density polyethylene (LDPE)
- High-density polyethylene (HDPE)
- Polypropylene (PP)
- Polyvinyl chloride (PVC)
- Polystyrene (PS)
- Nylon, nylon 6, nylon 6,6
- Teflon (Polytetrafluoroethylene)
- Thermoplastic polyurethanes (TPU)
| Polymer | Abbreviation | Properties | Uses |
|---|---|---|---|
| Low-density polyethylene | LDPE | Chemically inert, flexible, insulator | Squeeze bottles, toys, flexible pipes, insulation cover (electric wires), six pack rings, etc. |
| High-density polyethylene | HDPE | Inert, thermally stable, tough and high tensile strength | Bottles, pipes, inner insulation (dielectric) of coax cable (see also PTFE), plastic bags, etc. |
| Polypropylene | PP | Resistant to acids and alkalies, High tensile strength | Auto parts, industrial fibers, food containers, liner in bags, dishware and as a wrapping material for textiles and food |
| Polystyrene (thermocole) | PS | Thermal insulator. Properties depends on the form, expanded form is tough and rigid | Petri dishes, CD case, plastic cutlery |
| Polytetrafluoroethylene | PTFE | Very low coefficient of friction, excellent dielectric properties, chemically inert | Low friction bearings, non-stick pans, inner insulation (dielectric) of coax cable (see also HDPE), coating against chemical attack etc. |
| Polyvinyl chloride | PVC | Insulator, flame retardant, chemically inert | Pipe (mainly draining), fencing, lawn chairs, hand-bags, curtain clothes, non-food bottles, raincoats, toys, vinyl flooring, electrical installation insulations, etc. |
| Polychlorotrifluoroethylene | PCTFE | Stable to heat and thermal attacks, high tensile strength and non wetting | valves, seals, gaskets etc. |
Brand names
These polymers are often better known through their brand names, for instance:
| Brand Name | Polymer | Characteristic properties | Uses |
|---|---|---|---|
| Bakelite | Phenol-formaldehyde resin | High electric, heat and chemical resistance | Insulation of wires, manufacturing sockets, electrical devices, brake pads, etc. |
| Kevlar | Para-aramid fibre | High tensile strength | Manufacturing armour, sports and musical equipment. Used in the field of cryogenics |
| Twaron | Para-aramid | Heat resistant and strong fibre | Bullet-proof body armor, helmets, brake pads, ropes, cables and optical fibre cables, etc. and as an asbestos substitute |
| Mylar | Polyethylene terephthalate film | High strength and stiffness, less permeable to gases, almost reflects light completely | Food packaging, transparent covering over paper, reflector for rollsigns and solar cooking stoves |
| Neoprene | Polychloroprene | Chemically inert | Manufacturing gaskets, corrosion resistant coatings, waterproof seat covers, substitute for corks and latex |
| Nylon | Polyamide | Silky, thermoplastic and resistant to biological and chemical agents | Stockings, fabrics, toothbrushes. Molded nylon is used in making machine screws, gears etc. |
| Nomex | Meta-aramid polymer | Excellent thermal, chemical, and radiation resistance, rigid, durable and fireproof. | Hood of firefighter's mask, electrical lamination of circuit boards and transformer cores and in Thermal Micrometeoroid Garment |
| Orlon | Polyacrylonitrile (PAN) | Wool-like, resistant to chemicals, oils, moths and sunlight | Used for making clothes and fabrics like sweaters, hats, yarns, rugs, etc., and as a precursor of carbon fibres |
| Rilsan | Polyamide 11 & 12 | Bioplastic | Used in high-performance applications such as sports shoes, electronic device components, automotive fuel lines, pneumatic airbrake tubing, oil and gas flexible pipes and control fluid umbilicals, and catheters. |
| Technora | Copolyamid | High tensile strength, resistance to corrosion, heat, chemicals and saltwater | Used for manufacturing optical fiber cables, umbilical cables, drumheads, automotive industry, ropes, wire ropes and cables |
| Teflon | Polytetrafluoroethylene (PTFE) | Very low coefficient of friction, excellent dielectric properties, high melting, chemically inert | Plain bearings, gears, non-stick pans, etc. due to its low friction. Used as a tubing for highly corrosive chemicals. |
| Ultem | Polyimide | Heat,flame and solvent resistant. Has high dielectric strength | Used in medical and chemical instrumentation, also in guitar picks |
| Vectran | aromatic polyester | High thermal and chemical stability. Golden color. Has high strength, low creep, and is moisture resistant | Used as reinforcing fibres for ropes, cables, sailcloth. Also used in manufacturing badminton strings, bike tires and in electronics applications. Is the key component of a line of inflatable spacecraft developed by Bigelow Aerospace |
| Viton | Polytetrafluoroethylene (PTFE) | Elastomer | Depends on the grade of the polymer. Viton B is used in chemical process plants and gaskets. |
| Zylon | poly-p-phenylene-2,6-benzobisoxazole (PBO) | Very high tensile strength and thermal stability | Used in tennis racquets, table tennis blades, body armor, etc. |
Summary Chart
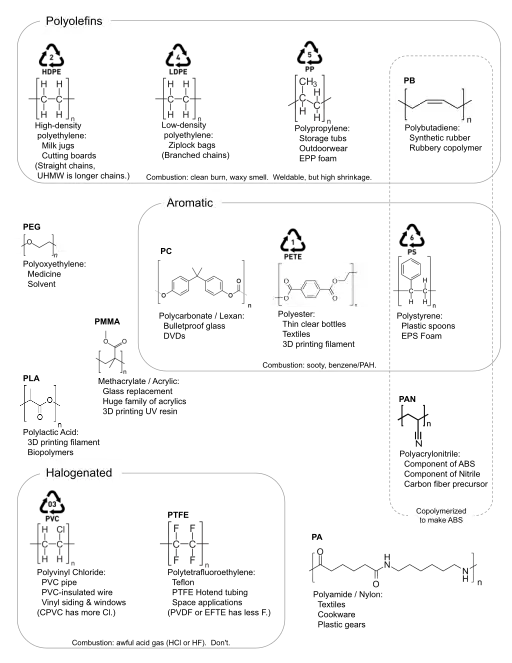
Plastic identification codes
References
- Andrew J. Peacock; Allison R. Calhoun (30 June 2006). Polymer Chemistry: Properties and Applications. Hanser Verlag. pp. 1–. ISBN 978-1-56990-397-1. Retrieved 15 July 2012.
- Srikanth Pilla (15 September 2011). Handbook of Bioplastics and Biocomposites Engineering Applications. John Wiley & Sons. p. 154. ISBN 978-1-118-17704-4. Retrieved 15 July 2012.
- "Glossary of Basic Terms in Polymer Science". Pure and Applied Chemistry. 68 (12): 2287–2301. 1996. doi:10.1351/goldbook.A00250. ISBN 978-0-9678550-9-7.