URB754
URB754 was originally reported by Piomelli et al. to be a potent, noncompetitive inhibitor of monoacylglycerol lipase (MGL).[1] However, recent studies have shown that URB754 failed to inhibit recombinant MGL, and brain FAAH activity was also resistant to URB754.[2] In a later study by Piomelli et al., the MGL-inhibitory activity attributed to URB754 is in fact due to a chemical impurity present in the commercial sample, identified as bis(methylthio)mercurane.[3]
 | |
| Names | |
|---|---|
| Preferred IUPAC name
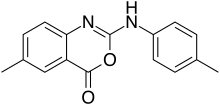
6-Methyl-2-(4-methylanilino)-4H-3,1-benzoxazin-4-one | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.236.075 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C16H14N2O2 | |
| Molar mass | 266.300 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
References
- Makara JK, Mor M, Fegley D, Szabó SI, Kathuria S, Astarita G, Duranti A, Tontini A, Tarzia G, Rivara S, Freund TF, Piomelli D (2005). "Selective inhibition of 2-AG hydrolysis enhances endocannabinoid signaling in hippocampus". Nat. Neurosci. 8 (9): 1139–41. doi:10.1038/nn1521. PMID 16116451. S2CID 52810445.
- Saario SM, Palomäki V, Lehtonen M, Nevalainen T, Järvinen T, Laitinen JT (2006). "URB754 has no effect on the hydrolysis or signaling capacity of 2-AG in the rat brain". Chem. Biol. 13 (8): 811–4. doi:10.1016/j.chembiol.2006.07.008. PMID 16931330.
- Tarzia, Giorgio; Antonietti, Francesca; Duranti, Andrea; Tontini, Andrea; Mor, Marco; Rivara, Silvia; Traldi, Pietro; Astarita, Giuseppe; King, Alvin; Clapper, Jason R.; Piomelli, Daniele (2007). "Identification of a Bioactive Impurity in a Commercial Sample of 6-Methyl-2-p-Tolylaminobenzo[d][1,3]Oxazin-4-One (URB754)". Annali di Chimica. 97 (9): 887–94. doi:10.1002/adic.200790073. PMID 17970304.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.