Ugi reaction
In organic chemistry, the Ugi reaction is a multi-component reaction involving a ketone or aldehyde, an amine, an isocyanide and a carboxylic acid to form a bis-amide.[1][2][3][4] The reaction is named after Ivar Karl Ugi, who first reported this reaction in 1959.
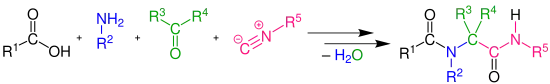
| Ugi reaction | |
|---|---|
| Named after | Ivar Karl Ugi |
| Reaction type | Coupling reaction |
| Identifiers | |
| Organic Chemistry Portal | ugi-reaction |
| RSC ontology ID | RXNO:0000129 |
The Ugi reaction is exothermic and usually complete within minutes of adding the isocyanide. High concentration (0.5M - 2.0M) of reactants give the highest yields. Polar, aprotic solvents, like DMF, work well. However, methanol and ethanol have also been used successfully. This uncatalyzed reaction has an inherent high atom economy as only a molecule of water is lost, and the chemical yield in general is high. Several reviews have been published.[5][6][7][8][9][10][11][12]
Due to the reaction products being potential protein mimetics there have been many attempts to development an enantioselective Ugi reaction,[13] the first successful report of which was in 2018.[14]
Reaction mechanism
One plausible reaction mechanism is depicted below:[15]
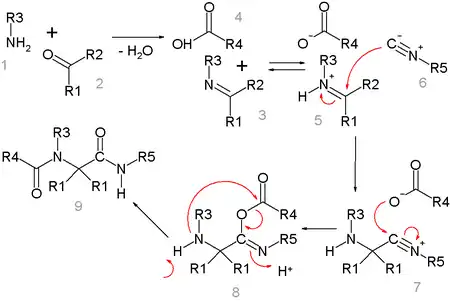
Amine 1 and ketone 2 form the imine 3 with loss of one equivalent of water. Proton exchange with carboxylic acid 4 activates the iminium ion 5 for nucleophilic addition of the isocyanide 6 with its terminal carbon atom to nitrilium ion 7. A second nucleophilic addition takes place at this intermediate with the carboxylic acid anion to 8. The final step is a Mumm rearrangement with transfer of the R4 acyl group from oxygen to nitrogen. All reaction steps are reversible except for the Mumm rearrangement, which drives the whole reaction sequence.
In the related Passerini reaction (lacking the amine) the isocyanide reacts directly with the carbonyl group but other aspects of the reaction are the same. This reaction can take place concurrently with the Ugi reaction, acting as a source of impurities.
Variations
Combination of reaction components
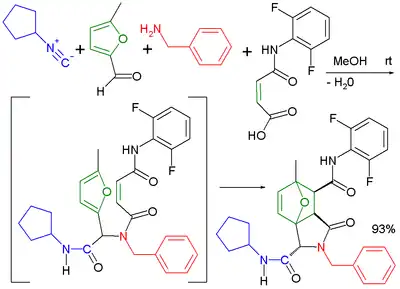
The usage of bifunctional reaction components greatly increases the diversity of possible reaction products. Likewise, several combinations lead to structurally interesting products. The Ugi reaction has been applied in combination with an intramolecular Diels-Alder reaction[16] in an extended multistep reaction.
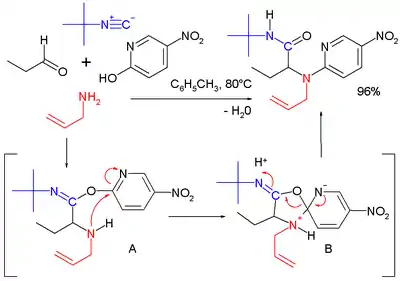
A reaction in its own right is the Ugi–Smiles reaction with the carboxylic acid component replaced by a phenol. In this reaction the Mumm rearrangement in the final step is replaced by the Smiles rearrangement.[17]
 |  | |
| Ugi–Diels–Alder reaction | Ugi–Smiles reaction | |
Another combination (with separate workup of the Ugi intermediate) is one with the Buchwald–Hartwig reaction.[18] In the Ugi–Heck reaction a Heck aryl-aryl coupling takes place in a second step.[19]
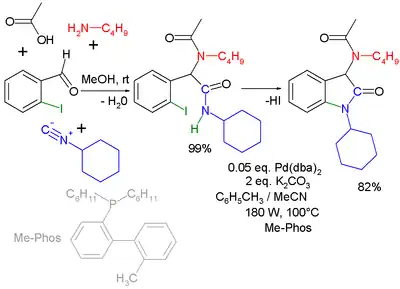 | 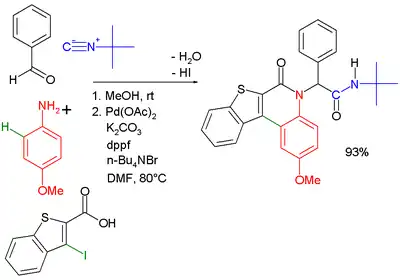 | |
| Ugi–Buchwald–Hartwig reaction [20] | Ugi–Heck reaction [21] | |
Combination of amine and carboxylic acid
Several groups have used β-amino acids in the Ugi reaction to prepare β-lactams.[22] This approach relies on acyl transfer in the Mumm rearrangement to form the four-membered ring. The reaction proceeds in moderate yield at room temperature in methanol with formaldehyde or a variety of aryl aldehydes. For example, p-nitrobenzaldehyde reacts to form the β-lactam shown in 71% yield as a 4:1 diastereomeric mixture:
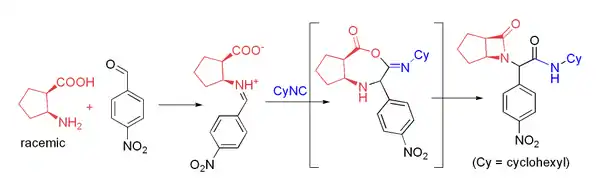
Applications
Chemical libraries
The Ugi reaction is one of the first reactions to be exploited explicitly to develop chemical libraries. These chemical libraries are sets of compounds that can be tested repeatedly. Using the principles of combinatorial chemistry, the Ugi reaction offers the possibility to synthesize a great number of compounds in one reaction, by the reaction of various ketones (or aldehydes), amines, isocyanides and carboxylic acids. These libraries can then be tested with enzymes or living organisms to find new active pharmaceutical substances. One drawback is the lack of chemical diversity of the products. Using the Ugi reaction in combination with other reactions enlarges the chemical diversity of possible products.
Examples of Ugi reaction combinations:
- Isoquinolines from Ugi and Heck reactions.[25]
Pharmaceutical industry
Crixivan can be prepared using the Ugi reaction.[26]
Additionally, many of the caine-type anesthetics are synthesized using this reaction. Examples include lidocaine and bupivacaine.
See also
References
- Ugi I, Meyr R, Fetzer U, Steinbrückner C (1959). "Versuche mit Isonitrilen". Angew. Chem. 71 (11): 386. doi:10.1002/ange.19590711110.
- Ugi I, Steinbrückner C (1960). "Über ein neues Kondensations-Prinzip". Angew. Chem. 72 (7–8): 267–268. Bibcode:1960AngCh..72..267U. doi:10.1002/ange.19600720709.
- Ugi, I. (1962). "The α-Addition of Immonium Ions and Anions to Isonitriles Accompanied by Secondary Reactions". Angewandte Chemie International Edition in English. 1 (1): 8–21. doi:10.1002/anie.196200081.
- Boltjes A, Liu H, Liu H, Dömling A (2017). "Ugi Multicomponent Reaction". Org. Synth. 94: 54–65. doi:10.15227/orgsyn.094.0054.
- Tripolitsiotis, Nikolaos P.; Thomaidi, Maria; Neochoritis, Constantinos G. (2020-11-15). "The Ugi Three-Component Reaction; a Valuable Tool in Modern Organic Synthesis". European Journal of Organic Chemistry. 2020 (42): 6525–6554. doi:10.1002/ejoc.202001157. ISSN 1434-193X. S2CID 224890321.
- Ugi I, Lohberger S, Karl R (1991). "The Passerini and Ugi Reactions". Comprehensive Organic Synthesis. Vol. 2. Oxford: Pergamon. pp. 1083–1109. ISBN 0-08-040593-2.
- Ugi I, Werner B, Dömling A (2003). "The Chemistry of Isocyanides, their MultiComponent Reactions and their Libraries" (PDF). Molecules. 8: 53–66. doi:10.3390/80100053. S2CID 53949436.
- Banfi L, Riva R (2005). "The Passerini Reaction". In Overman LE (ed.). Organic Reactions. Vol. 65. Wiley. ISBN 0-471-68260-8.)
- Tempest PA (November 2005). "Recent advances in heterocycle generation using the efficient Ugi multiple-component condensation reaction". Current Opinion in Drug Discovery & Development. 8 (6): 776–88. PMID 16312152.
- Ugi I, Heck S (February 2001). "The multicomponent reactions and their libraries for natural and preparative chemistry". Combinatorial Chemistry & High Throughput Screening. 4 (1): 1–34. doi:10.2174/1386207013331291. PMID 11281825.
- Bienayme H, Hulme C, Oddon G, Schmitt P (September 2000). "Maximizing synthetic efficiency: multi-component transformations lead the way". Chemistry: A European Journal. 6 (18): 3321–9. doi:10.1002/1521-3765(20000915)6:18<3321::AID-CHEM3321>3.0.CO;2-A. PMID 11039522.
- Dömling A, Ugi I (September 2000). "Multicomponent Reactions with Isocyanides". Angewandte Chemie. 39 (18): 3168–3210. doi:10.1002/1521-3773(20000915)39:18<3168::AID-ANIE3168>3.0.CO;2-U. PMID 11028061.
- Wang Q, Wang DX, Wang MX, Zhu J (May 2018). "Still Unconquered: Enantioselective Passerini and Ugi Multicomponent Reactions". Accounts of Chemical Research. 51 (5): 1290–1300. doi:10.1021/acs.accounts.8b00105. PMID 29708723.
- Zhang J, Yu P, Li SY, Sun H, Xiang SH, Wang JJ, et al. (September 2018). "Asymmetric phosphoric acid-catalyzed four-component Ugi reaction". Science. 361 (6407): eaas8707. doi:10.1126/science.aas8707. PMID 30213886.
- Denmark SE, Fan Y (November 2005). "Catalytic, enantioselective alpha-additions of isocyanides: Lewis base catalyzed Passerini-type reactions". The Journal of Organic Chemistry. 70 (24): 9667–76. doi:10.1021/jo050549m. PMID 16292793.
- Ilyin A, Kysil V, Krasavin M, Kurashvili I, Ivachtchenko AV (December 2006). "Complexity-enhancing acid-promoted rearrangement of tricyclic products of tandem Ugi 4CC/intramolecular Diels-Alder reaction". The Journal of Organic Chemistry. 71 (25): 9544–7. doi:10.1021/jo061825f. PMID 17137394.
- El Kaim L, Gizolme M, Grimaud L, Oble J (August 2006). "Direct access to heterocyclic scaffolds by new multicomponent Ugi-Smiles couplings". Organic Letters. 8 (18): 4019–21. doi:10.1021/ol061605o. PMID 16928063.
- Bonnaterre F, Bois-Choussy M, Zhu J (September 2006). "Rapid access to oxindoles by the combined use of an Ugi four-component reaction and a microwave-assisted intramolecular Buchwald-Hartwig amidation reaction". Organic Letters. 8 (19): 4351–4. doi:10.1021/ol061755z. PMID 16956224.
- Ma Z, Xiang Z, Luo T, Lu K, Xu Z, Chen J, Yang Z (2006). "Synthesis of functionalized quinolines via Ugi and Pd-catalyzed intramolecular arylation reactions". Journal of Combinatorial Chemistry. 8 (5): 696–704. doi:10.1021/cc060066b. PMID 16961408.
- Second part microwave accelerated reaction with Pd(dba)2 and phosphine ligand Me-Phos
- The Heck step takes place with palladium(II) acetate, dppf ligand potassium carbonate and tetra-n-butylammonium bromide in dimethylformamide
- Gedey S, Van der Eycken J, Fülöp F (May 2002). "Liquid-phase combinatorial synthesis of alicyclic beta-lactams via Ugi four-component reaction". Organic Letters. 4 (11): 1967–9. doi:10.1021/ol025986r. PMID 12027659.
- Zhang J, Jacobson A, Rusche JR, Herlihy W (February 1999). "Unique Structures Generated by Ugi 3CC Reactions Using Bifunctional Starting Materials Containing Aldehyde and Carboxylic Acid". The Journal of Organic Chemistry. 64 (3): 1074–1076. doi:10.1021/jo982192a. PMID 11674195.
- Short KM, Mjalli AM (1997). "A solid-phase combinatorial method for the synthesis of novel 5- and 6-membered ring lactams". Tetrahedron Letters. 38 (3): 359–362. doi:10.1016/S0040-4039(96)02303-9.
- Xiang Z, Luo T, Lu K, Cui J, Shi X, Fathi R, et al. (September 2004). "Concise synthesis of isoquinoline via the Ugi and Heck reactions". Organic Letters. 6 (18): 3155–8. doi:10.1021/ol048791n. PMID 15330611.
- Rossen K, Pye PJ, DiMichele LM, Volante RP, Reider PJ (1998). "An efficient asymmetric hydrogenation approach to the synthesis of the Crixivan piperazine intermediate". Tetrahedron Letters. 39 (38): 6823–6826. doi:10.1016/S0040-4039(98)01484-1.