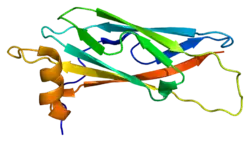VAPA
VAMP-Associated Protein A ( or Vesicle-Associated Membrane Protein-Associated Protein A) is a protein that in humans is encoded by the VAPA gene.[5][6][7] Together with VAPB and VAPC it forms the VAP protein family. They are integral endoplasmic reticulum membrane proteins of the type II and are ubiquitous among eukaryotes.[8]
VAPA is ubiquitously expressed in human tissues[5] and is thought to be involved in membrane trafficking by interaction with SNAREs,[9] in regulation of lipid transport and metabolism,[8] and in the Unfolded Protein Response (UPR).[8]
Protein structure
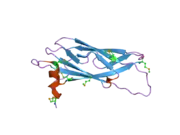
The protein is divided in three different domains.[5] First, an N-terminal beta-sheet with an immunoglobulin-like fold that shares homology with the Nematode major sperm protein (MSP). Secondly, a central coiled-coil domain. Then finally a C-terminal transmembrane domain (TMD) which is usually present in proteins of the t-SNARE superfamily and has been found in other proteins associated with vesicular transport.[10] VAPA can form homo-dimers and also hetero dimers with VAPB by interactions through their (TMD).[5]
Intracellular Localisation
Because of its ubiquitous expression,[5] the intracellular localisation and function of VAPA may vary between cell types. It is however mainly located in the ER,[11] Golgi apparatus and the Vesicular Tubular Compartment or ER-Golgi Intermediate Compartment,[9] an organelle of eukaryotic cells consisting in fused ER-derived vesicles that transports proteins from the ER to the Golgi apparatus.[12]
Interactions
VAPA has been documented to interact with three different groups of proteins: proteins associated with vesicle traffic and fusion, proteins containing the FFAT motif and viral proteins.[8]
Vesicle traffic and fusion
VAPA is able to bind a range of SNARE proteins including syntaxin1A, rbet1 and rsec22. It also binds to proteins associated with membrane fusion machinery such as alphaSNAP and NSF.These interaction suggest that VAPA could have a general role in the regulation of the function of these proteins that are mainly involved in membrane fusion[9]
Viral Proteins
VAP proteins have been found to be essential host factors for several viruses.[13][14][15]
VAP proteins binds with non-structural proteins of the hepatitis C virus NS5A and NS5B allowing the RNA replication machinery of the virus to set up on the lipid raft membrane of the host cell.[16]
VAPA also binds to several viral proteins from the Norovirus family and is important for the virus replication efficiency.[13][14] The non-structural proteins NS1 and NS2 are able to bind VAPA thanks to sequence mimicry of the FFAT motif probably yielding the same advantage to viral replication as for hepatitis C virus.[14]
Function
One of its proposed functions is to slow down the lipid flow back towards the ER when protein misfolding occurs, in order to reduce the amount of stress triggered by the UPR. The VAP would regulate this process by inhibiting membrane contact.[19]
Associated Diseases
The P56S SNP in the MSP domain of VAPB is involved in the onset of Lou Gehrig's disease also called amyotrophic lateral sclerosis (ALS) where the patient loses muscle control and function. The degenerescence of motor neurons observed in such condition could to be due to the inability of VAPB to regulate the lipid function around the ER and the subsequent consequences on cell function.[19]
References
- GRCh38: Ensembl release 89: ENSG00000101558 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000024091 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- Nishimura Y, Hayashi M, Inada H, Tanaka T (January 1999). "Molecular cloning and characterization of mammalian homologues of vesicle-associated membrane protein-associated (VAMP-associated) proteins". Biochemical and Biophysical Research Communications. 254 (1): 21–6. doi:10.1006/bbrc.1998.9876. PMID 9920726.
- Weir ML, Klip A, Trimble WS (July 1998). "Identification of a human homologue of the vesicle-associated membrane protein (VAMP)-associated protein of 33 kDa (VAP-33): a broadly expressed protein that binds to VAMP". The Biochemical Journal. 333 ( Pt 2) (2): 247–51. doi:10.1042/bj3330247. PMC 1219579. PMID 9657962.
- "Entrez Gene: VAPA VAMP (vesicle-associated membrane protein)-associated protein A, 33kDa".
- Lev S, Ben Halevy D, Peretti D, Dahan N (June 2008). "The VAP protein family: from cellular functions to motor neuron disease". Trends in Cell Biology. 18 (6): 282–90. doi:10.1016/j.tcb.2008.03.006. PMID 18468439.
- Weir ML, Xie H, Klip A, Trimble WS (August 2001). "VAP-A binds promiscuously to both v- and tSNAREs". Biochemical and Biophysical Research Communications. 286 (3): 616–21. doi:10.1006/bbrc.2001.5437. PMID 11511104.
- Weimbs T, Low SH, Chapin SJ, Mostov KE, Bucher P, Hofmann K (April 1997). "A conserved domain is present in different families of vesicular fusion proteins: a new superfamily". Proceedings of the National Academy of Sciences of the United States of America. 94 (7): 3046–51. doi:10.1073/pnas.94.7.3046. PMC 20319. PMID 9096343.
- Skehel PA, Fabian-Fine R, Kandel ER (February 2000). "Mouse VAP33 is associated with the endoplasmic reticulum and microtubules". Proceedings of the National Academy of Sciences of the United States of America. 97 (3): 1101–6. doi:10.1073/pnas.97.3.1101. PMC 15535. PMID 10655491.
- Appenzeller-Herzog C, Hauri HP (June 2006). "The ER-Golgi intermediate compartment (ERGIC): in search of its identity and function". Journal of Cell Science. 119 (Pt 11): 2173–83. doi:10.1242/jcs.03019. PMID 16723730.
- Ettayebi K, Hardy ME (November 2003). "Norwalk virus nonstructural protein p48 forms a complex with the SNARE regulator VAP-A and prevents cell surface expression of vesicular stomatitis virus G protein". Journal of Virology. 77 (21): 11790–7. doi:10.1128/JVI.77.21.11790-11797.2003. PMC 229264. PMID 14557663.
- McCune BT, Tang W, Lu J, Eaglesham JB, Thorne L, Mayer AE, Condiff E, Nice TJ, Goodfellow I, Krezel AM, Virgin HW (July 2017). "Noroviruses Co-opt the Function of Host Proteins VAPA and VAPB for Replication via a Phenylalanine-Phenylalanine-Acidic-Tract-Motif Mimic in Nonstructural Viral Protein NS1/2". mBio. 8 (4): e00668–17. doi:10.1128/mBio.00668-17. PMC 5513711. PMID 28698274.
- Ishikawa-Sasaki K, Nagashima S, Taniguchi K, Sasaki J (April 2018). "Model of OSBP-Mediated Cholesterol Supply to Aichi Virus RNA Replication Sites Involving Protein-Protein Interactions among Viral Proteins, ACBD3, OSBP, VAP-A/B, and SAC1". Journal of Virology. 92 (8): e01952–17. doi:10.1128/JVI.01952-17. PMC 5874406. PMID 29367253.
- Gao L, Aizaki H, He JW, Lai MM (April 2004). "Interactions between viral nonstructural proteins and host protein hVAP-33 mediate the formation of hepatitis C virus RNA replication complex on lipid raft". Journal of Virology. 78 (7): 3480–8. doi:10.1128/JVI.78.7.3480-3488.2004. PMC 371042. PMID 15016871.
- Wyles JP, McMaster CR, Ridgway ND (August 2002). "Vesicle-associated membrane protein-associated protein-A (VAP-A) interacts with the oxysterol-binding protein to modify export from the endoplasmic reticulum". The Journal of Biological Chemistry. 277 (33): 29908–18. doi:10.1074/jbc.M201191200. PMID 12023275.
- Wyles JP, Ridgway ND (July 2004). "VAMP-associated protein-A regulates partitioning of oxysterol-binding protein-related protein-9 between the endoplasmic reticulum and Golgi apparatus". Experimental Cell Research. 297 (2): 533–47. doi:10.1016/j.yexcr.2004.03.052. PMID 15212954.
- Ernst WL, Shome K, Wu CC, Gong X, Frizzell RA, Aridor M (March 2016). "VAMP-associated Proteins (VAP) as Receptors That Couple Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Proteostasis with Lipid Homeostasis". The Journal of Biological Chemistry. 291 (10): 5206–20. doi:10.1074/jbc.M115.692749. PMC 4777854. PMID 26740627.
Further reading
- Lapierre LA, Tuma PL, Navarre J, Goldenring JR, Anderson JM (November 1999). "VAP-33 localizes to both an intracellular vesicle population and with occludin at the tight junction". Journal of Cell Science. 112 ( Pt 21) (21): 3723–32. doi:10.1242/jcs.112.21.3723. PMID 10523508.
- Tu H, Gao L, Shi ST, Taylor DR, Yang T, Mircheff AK, Wen Y, Gorbalenya AE, Hwang SB, Lai MM (October 1999). "Hepatitis C virus RNA polymerase and NS5A complex with a SNARE-like protein". Virology. 263 (1): 30–41. doi:10.1006/viro.1999.9893. PMID 10544080.
- Weir ML, Xie H, Klip A, Trimble WS (August 2001). "VAP-A binds promiscuously to both v- and tSNAREs". Biochemical and Biophysical Research Communications. 286 (3): 616–21. doi:10.1006/bbrc.2001.5437. PMID 11511104.
- Wyles JP, McMaster CR, Ridgway ND (August 2002). "Vesicle-associated membrane protein-associated protein-A (VAP-A) interacts with the oxysterol-binding protein to modify export from the endoplasmic reticulum". The Journal of Biological Chemistry. 277 (33): 29908–18. doi:10.1074/jbc.M201191200. PMID 12023275.
- Matsuda A, Suzuki Y, Honda G, Muramatsu S, Matsuzaki O, Nagano Y, Doi T, Shimotohno K, Harada T, Nishida E, Hayashi H, Sugano S (May 2003). "Large-scale identification and characterization of human genes that activate NF-kappaB and MAPK signaling pathways". Oncogene. 22 (21): 3307–18. doi:10.1038/sj.onc.1206406. PMID 12761501.
- Gao L, Aizaki H, He JW, Lai MM (April 2004). "Interactions between viral nonstructural proteins and host protein hVAP-33 mediate the formation of hepatitis C virus RNA replication complex on lipid raft". Journal of Virology. 78 (7): 3480–8. doi:10.1128/JVI.78.7.3480-3488.2004. PMC 371042. PMID 15016871.
- Evans MJ, Rice CM, Goff SP (August 2004). "Phosphorylation of hepatitis C virus nonstructural protein 5A modulates its protein interactions and viral RNA replication". Proceedings of the National Academy of Sciences of the United States of America. 101 (35): 13038–43. Bibcode:2004PNAS..10113038E. doi:10.1073/pnas.0405152101. PMC 516513. PMID 15326295.
- Suzuki Y, Yamashita R, Shirota M, Sakakibara Y, Chiba J, Mizushima-Sugano J, Nakai K, Sugano S (September 2004). "Sequence comparison of human and mouse genes reveals a homologous block structure in the promoter regions". Genome Research. 14 (9): 1711–8. doi:10.1101/gr.2435604. PMC 515316. PMID 15342556.
- Rual JF, Venkatesan K, Hao T, Hirozane-Kishikawa T, Dricot A, Li N, Berriz GF, Gibbons FD, Dreze M, Ayivi-Guedehoussou N, Klitgord N, Simon C, Boxem M, Milstein S, Rosenberg J, Goldberg DS, Zhang LV, Wong SL, Franklin G, Li S, Albala JS, Lim J, Fraughton C, Llamosas E, Cevik S, Bex C, Lamesch P, Sikorski RS, Vandenhaute J, Zoghbi HY, Smolyar A, Bosak S, Sequerra R, Doucette-Stamm L, Cusick ME, Hill DE, Roth FP, Vidal M (October 2005). "Towards a proteome-scale map of the human protein-protein interaction network". Nature. 437 (7062): 1173–8. Bibcode:2005Natur.437.1173R. doi:10.1038/nature04209. PMID 16189514. S2CID 4427026.
- Hamamoto I, Nishimura Y, Okamoto T, Aizaki H, Liu M, Mori Y, Abe T, Suzuki T, Lai MM, Miyamura T, Moriishi K, Matsuura Y (November 2005). "Human VAP-B is involved in hepatitis C virus replication through interaction with NS5A and NS5B". Journal of Virology. 79 (21): 13473–82. doi:10.1128/JVI.79.21.13473-13482.2005. PMC 1262604. PMID 16227268.
- Kawano M, Kumagai K, Nishijima M, Hanada K (October 2006). "Efficient trafficking of ceramide from the endoplasmic reticulum to the Golgi apparatus requires a VAMP-associated protein-interacting FFAT motif of CERT". The Journal of Biological Chemistry. 281 (40): 30279–88. doi:10.1074/jbc.M605032200. PMID 16895911.