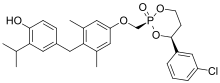
VK2809
VK2809 (formerly known as MB07811) is a thyromimetic prodrug whose active form is selective for the THR-β isoform. It is being developed by Viking Therapeutics in a phase II trial for the treatment of nonalcoholic steatohepatitis[1][2] and is also being investigated for glycogen storage disease type Ia.[3]
 | |
| Clinical data | |
|---|---|
| Other names | VK-2809; MB07811 |
| Identifiers | |
| |
| CAS Number | |
| DrugBank | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C28H32ClO5P |
| Molar mass | 514.98 g·mol−1 |
References
- "VK2809". Viking Therapeutics. Retrieved 15 September 2023.
- Alkhouri, Naim (1 February 2020). "Thyromimetics as emerging therapeutic agents for nonalcoholic steatohepatitis: rationale for the development of resmetirom (MGL-3196)". Expert Opinion on Investigational Drugs. 29 (2): 99–101. doi:10.1080/13543784.2020.1708899. ISSN 1354-3784.
- Zhou, Jin; Waskowicz, Lauren R.; Lim, Andrea; Liao, Xiao-Hui; Lian, Brian; Masamune, Hiroko; Refetoff, Samuel; Tran, Brian; Koeberl, Dwight D.; Yen, Paul M. (1 August 2019). "A Liver-Specific Thyromimetic, VK2809, Decreases Hepatosteatosis in Glycogen Storage Disease Type Ia". Thyroid. 29 (8): 1158–1167. doi:10.1089/thy.2019.0007. ISSN 1050-7256. PMC 6707038. PMID 31337282.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.