Varroa destructor
Varroa destructor, the Varroa mite is an external parasitic mite that attacks and feeds on honey bees and is one of the most severe honeybee pests in the world.[2][3] A significant mite infestation leads to the death of a honey bee colony, usually in the late autumn through early spring. Without management for Varroa mite, honey bee colonies typically collapse within 2 to 3 years in temperate climates.[4] These mites can infest the western honeybee Apis mellifera and the eastern honeybee Apis cerana.
| Varroa mite | |
|---|---|
.jpg.webp) | |
.jpg.webp) | |
| Varroa destructor adult female in dorsal (top) and ventral (lower) views | |
| Scientific classification | |
| Domain: | Eukaryota |
| Kingdom: | Animalia |
| Phylum: | Arthropoda |
| Subphylum: | Chelicerata |
| Class: | Arachnida |
| Order: | Mesostigmata |
| Family: | Varroidae |
| Genus: | Varroa |
| Species: | V. destructor |
| Binomial name | |
| Varroa destructor Anderson & Trueman, 2000[1] | |
The parasitism of bees by mites in the genus Varroa is called varroosis. The Varroa mite can reproduce only in a honey bee colony. It attaches to the body of the bee and weakens the bee by sucking fat bodies.[5] The species is a vector for at least five debilitating bee viruses,[5] including RNA viruses such as the deformed wing virus (DWV). The Varroa mite is the parasite with possibly the most pronounced economic impact on the beekeeping industry. Varroa is considered to be one of multiple stress factors[6] contributing to the higher levels of bee losses around the world.
Identification
The adult female mite is reddish-brown in color, while the male is white. Varroa mites are flat, having a button shape. They are 1–1.8 mm long and 1.5–2 mm wide, and have eight legs.[7] Varroa mites lack eyes.[8]
Until 2000, V. destructor was thought to be a closely related mite species called Varroa jacobsoni and resulted in some mislabeling in the scientific literature.[1] Because of this, most pre-2000 research that refers to V. jacobsoni was actually research on V. destructor.[4] Both species parasitize the eastern honey bee, A. cerana. However, the species originally described as V. jacobsoni by Anthonie Cornelis Oudemans in 1904 does not attack the western honey bee, unlike V. destructor. Otherwise, the species can only be distinguished by DNA analysis[1][9]
.jpg.webp) V. destructor protonymph
V. destructor protonymph Deutonymph of V. destructor
Deutonymph of V. destructor_(5048079279).jpg.webp) V. destructor adult male
V. destructor adult male.jpg.webp) Adult female in lateral view
Adult female in lateral view
Range
Varroa mites originally only occurred in Asia, on the Asian honeybee, Apis cerana, but this species has been introduced to many other countries on several continents, resulting in disastrous infestations of European honeybees.[10]
Introduction data prior to 2000 is unclear due to confusion with V. jacobsoni. By 2020, V. destructor was confirmed to be present throughout North America excluding Greenland, South America, most of Europe and Asia, and portions of Africa. The species was not present in Australia as well as Oman, Congo, Democratic Republic of Congo, and Malawi. It was suspected to not be present in Sudan and Somalia.[11][12] Mites were found in 2022 in New South Wales in Australia.[13]
Life cycle
Mites reproduce on a 10-day cycle. The female mite enters a honey bee brood cell. As soon as the cell is capped, the Varroa mite lays eggs on the larva. The young mites, typically several females and one male, hatch in about the same time as the young bee develops and leave the cell with the host. When the young bee emerges from the cell after pupation, the Varroa mites also leave and spread to other bees and larvae. The mite preferentially infest drone cells, allowing the mite to reproduce one more time with the extra three days it takes a drone to emerge compared to a worker bee. This can cause genetic defects such as useless wings or viruses and fungi in the bee.
Behavior
Adult mites suck on the fat body of both adult bees and bee larvae for sustenance. As the fat body is crucial for many bodily functions such as hormone and energy regulation, immunity, and pesticide detoxification, the bee is left in a severely weakened state. Adult mites live and feed under the abdominal plates of adult bees primarily on the underside of the metasoma region on the left side of the bee. Adult mites are more often identified as present in the hive when on top of the adult bee on the mesosoma region, but research suggests that mites in this location are not feeding, but rather attempting to transfer to another bee.[5]

Open wounds left by the feeding become sites for disease and virus infections. The mites are vectors for at least five and possibly up to 18 debilitating bee viruses,[5] including RNA viruses such as the deformed wing virus. With the exception of some resistance in the Russian strains and bees that have Varroa-sensitive hygiene (about 10% of colonies naturally have it), European Apis mellifera bees are almost completely defenseless against these parasites. (Russian honey bees are one-third to one-half less susceptible to mite reproduction).[14]
Mite populations undergo exponential growth when bee broods are available, and exponential decline when no brood is available. In 12 weeks, the number of mites in a western honey bee hive can multiply by (roughly) 12. Mites often invade colonies in the summer, leading to high mite populations in autumn.[15] High mite populations in the autumn can cause a crisis when drone rearing ceases and the mites switch to worker larvae, causing a quick population crash and often hive death.[16]
Once infected with a V. destructor mite, the honey bee may be damaged two ways. Firstly, the mite's consumption of the fat body weakens both the adult bee and the larva; in particular, it significantly decreases the weight of both the hatching and adult bee. Additionally, infected adult worker bees have a shorter lifespan than ordinary worker bees, and they furthermore tend to be absent from the colony far more than ordinary bees, which could be due to their reduced ability to navigate or regulate their energy for flight. Secondly, the mites are vectors of various viruses, in particular the deformed wing virus.[17]
After the initial developmental stages, when the young bee matures, it leaves the brood cell and takes the mite with it. V. destructor then leaves the young bee for an older one, preferably for a nurse bee, because nurse bees spend more time near the brood, giving the mite more ample opportunity to reproduce. In fact, because the nurse bee spends more time around the drone brood rather than the worker brood, many more drones are infected with the mites.[17]
Varroa mites have been found on tricial larvae of some wasp species, such as Vespula vulgaris, and flower-feeding insects such as the bumblebee, Bombus pensylvanicus, the scarab beetle, Phanaeus vindex, and the flower-fly, Palpada vinetorum.[18] It parasitizes the young larvae and feeds on the internal organs of the hosts. Although the Varroa mite cannot reproduce on these insects, its presence on them may be a means by which it spreads short distances (phoresy).
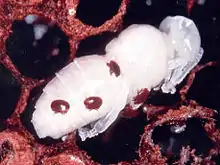
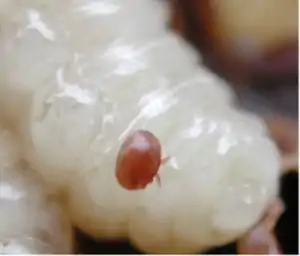
Virus transmission
Prior to the widespread introduction of Varroa mite, honey bee viruses were typically considered a minor issue. Virus particles are directly injected into the bee's body cavity and mites can also cause immunosuppression that increases infection in host bees. Varroa mites can transmit the following viruses:[4]
- Kashmir bee virus
- Sacbrood virus
- Acute bee paralysis virus
- Israeli acute paralysis virus
- Deformed wing virus
Deformed wing virus is one of the most prominent and damaging honeybee viruses transmitted by Varroa mites. It causes crumpled deformed wings that resemble sticks and also causes shortened abdomens.[4][19]

Colony collapse disorder
There is some evidence that harm from both Varroa mite and associated viruses they transmit may be a contributing factor that leads to colony collapse disorder (CCD).[2] While the exact causes of CCD are not known, infection of colonies from multiple pathogens and interaction of those pathogens with environmental stresses is considered by entomologists to be one of the likely causes of CCD.[20][21] Most scientists agree there is not a single cause of CCD.[22]
Management

Monitoring
Beekeepers use several methods for monitoring levels of Varroa mites in a colony.[23] They involve either estimating the total number of mites in a hive by using a sticky board under a screen bottom board to capture mites falling from the hive or estimating the number of mites per bee with powdered sugar or an ethanol wash.[24]
Monitoring for mites with a sticky board can be used to estimate the total number of mites in a colony over 72 hours using the equation:
where b is the number of mites found on the sticky board and c is the number of estimated mites in the colony. However, the bee population in a colony also needs to be known to determine what population of mites is tolerable with this method.[24]
Mite counts from a known quantity of bees (i.e., 300 bees) collected from brood comb are instead often used to determine mite severity. Mites are dislodged from a sample of bees using non-lethal or lethal means. The bees are shaken in a container of either powdered sugar, alcohol, or soapy water to dislodge and count mites. Powdered sugar is generally considered non-lethal to honey bees, but lethal methods such as alcohol can be more effective at dislodging mites.[25][24] 3% of the colony being infested is considered an economic threshold damaging enough to warrant further management such as miticides, though beekeepers may use other management tactics in the 0–2% infestation range to keep mite populations low.[24]
Chemical measures
Varroa mites can be treated with commercially available acaricides that must be timed carefully to minimize the contamination of honey that might be consumed by humans. The four most common synthetic pesticides used for mite treatments with formulations specific for honey bee colony use are amitraz, coumaphos, and two pyrethroids, flumethrin and (tau-fluvalinate), while naturally occurring compounds include formic acid, oxalic acid, essential oils such as thymol and beta acids from hops resin (e.g. lupulone). Many of these products whether synthetic or naturally produced can negatively affect honey bee brood or queens. These products often are applied through impregnated plastic strips or as powders spread between brood frames.[24]
Synthetic compounds often have high efficacy against Varroa mites, but resistance has occurred for all of these products in different areas of the world. Amitraz (e.g., Apivar strips) will typically kill 75–90% of mites, but is often not very affordable. Use of coumaphos has decreased over time due to low efficacy and resistance. Pyrethroids are used because a concentrations that will kill mites have relatively low toxicity to honey bees. Naturally-occurring compounds such as formic acid can range 35–75% mite mortality with efficacy varying based on temperature, brood population, and proximity to the chemical within the hive. Formic acid can also cause brood or queen mortality during high temperatures. Oxalic acid is used during broodless periods in the hive; it increases grooming behavior in bees and increases mite dislodging, and there are no known cases of Varroa mite resistance to this chemical.[24]
Compounds derived from plants have also been assessed for mite management. Thymol is one essential oil with efficacy against mites that varies, similar to formic acid, with temperature and brood population as well as being harmful to bees during high temperatures. Other essential oils such as garlic, oregano, and neem oil have had some efficacy in field trials, though most essential oils that have been tested have little to no effect. Essential oil use is widespread in hives with many of those uses being off-label or in violation of pesticide regulations in various countries. Hop beta acids are lupulones obtained from hop plants and have been used in products marketed for mite control. They have low toxicity to humans and bees, but efficacy is mixed ranging 43–88% mite mortality.[24]
Mechanical control
Varroa mites can also be controlled through nonchemical means. Most of these controls are intended to reduce the mite population to a manageable level, not to eliminate the mites completely.[24]
Screened bottom boards are used both for monitoring and can modestly reduce mite populations by 11–14%. Mites which fall from the comb or bees can land outside the hive instead landing on a solid bottom board that would allow them to easily return to the nest.[24]
Varroa infest drone cells at a higher rate than worker brood cells, so drone cells can be used as a trap for mite removal. Beekeepers can also introduce a frame with drone foundation cells that encourage bees to construct more drone cells. When the drone cells are capped, the frame can be removed to freeze out mites. This labor intensive process can reduce mite levels by about 50–93%, but if trap cells are not removed early enough before mites emerge, mite populations can spike. This method is only viable in spring and early summer when drones are produced.[24]
Heat is also sometimes used as a control method. The mites cannot survive temperatures near 40 °C (104 °F), but brief exposure to these temperatures do not harm honey bees. Devices are marketed intended to heat brood to these temperatures, though the efficacy of many of these products has not been reviewed.[24][26]
Powdered sugar used for estimating mite counts in hives has also been considered for mite management as it or other inert dusts were believed to initiate grooming responses. Long-term studies do not show any efficacy for reducing mite populations.[24]
Genetics
Researchers have been able to use RNA interference by feeding honey bees mixtures of double-stranded RNA that reduced Varroa mite infestation up to 50%.[27][28]
Efforts also have been made to breed hygienic honey bees, such as Varroa sensitive hygiene with resistance to Varroa mites.[29][30][31] Hygienic behaviors include:[29]
- Workers removing pupae heavily infested with mites, which kills both the developing bee and immature mites.
- An extended phoretic period in adult female mites.
- Grooming or removal from the brood cell increases adult mite mortality.
- Mites removed from host pupae are at an incorrect life stage to re-infest another pupa.
Hygienic behavior is effective against diseases such as American foulbrood or chalkbrood, but the efficacy of this behavior against mites is not quantified well; colonies with this behavior alone do not necessarily result in Varroa mite resistant colonies that can survive without miticide treatments. There are minimal trade-off costs to hives that have this behavior, so it is being actively pursued in bee breeding programs.[29]
See also
References
- D. L. Anderson & J. W. H. Trueman (2000). "Varroa jacobsoni (Acari: Varroidae) is more than one species". Experimental and Applied Acarology. 24 (3): 165–189. doi:10.1023/A:1006456720416. PMID 11108385. S2CID 12271915.
- Zakar, E.; Jávor, A.; Kusza, Sz. (August 2014). "Genetic bases of tolerance to Varroa destructor in honey bees (Apis mellifera L.)". Insectes Sociaux. 61 (3): 207–215. doi:10.1007/s00040-014-0347-5.
- Insect resistance management: biology, economics and prediction (1. ed.). Amsterdam: Elsevier/Acad. Press. 2008. p. 157. ISBN 978-0-12-373858-5.
- Rosenkranz, Peter; Aumeier, Pia; Ziegelmann, Bettina (January 2010). "Biology and control of Varroa destructor". Journal of Invertebrate Pathology. 103: S96–S119. doi:10.1016/j.jip.2009.07.016.
- Ramsey, Samuel D.; Ochoa, Ronald; Bauchan, Gary; Gulbronson, Connor; Mowery, Joseph D.; Cohen, Allen; Lim, David; Joklik, Judith; Cicero, Joseph M.; Ellis, James D.; Hawthorne, David; vanEngelsdorp, Dennis (29 January 2019). "Varroa destructor feeds primarily on honey bee fat body tissue and not hemolymph". Proceedings of the National Academy of Sciences. 116 (5): 1792–1801. Bibcode:2019PNAS..116.1792R. doi:10.1073/pnas.1818371116. PMC 6358713. PMID 30647116.
- Goulson, D.; Nicholls, E.; Botias, C.; Rotheray, E. L. (26 February 2015). "Bee declines driven by combined stress from parasites, pesticides, and lack of flowers" (PDF). Science. 347 (6229): 1255957. doi:10.1126/science.1255957. PMID 25721506. S2CID 206558985.
- "Varroa destructor : USDA ARS". www.ars.usda.gov. Archived from the original on June 28, 2023. Retrieved 2021-03-25.
- "Varroa Mite - Biology and Diagnosis". www.omafra.gov.on.ca. Archived from the original on August 21, 2023. Retrieved 2021-11-03.
- Maria J. Navajas (2010). "Tracking the colonisation history of the invasive species Varroa destructor". In Maurice Sabelis; Jan Bruin (eds.). Trends in Acarology. Proceedings of the 12th International Congress. Springer. pp. 375–378. doi:10.1007/978-90-481-9837-5_61. ISBN 978-90-481-9836-8.
- Invasion Biology Introduction: Varroa mites University of Columbia. Accessed 26 April 2017
- "Pathogens and pests". worldhoneybeehealth.com. Retrieved 27 August 2023.
- Boncristiani, Humberto; Ellis, James D.; Bustamante, Tomas; Graham, Jason; Jack, Cameron; Kimmel, Chase B.; Mortensen, Ashley; Schmehl, Daniel R. (2 January 2021). "World Honey Bee Health: The Global Distribution of Western Honey Bee ( Apis mellifera L.) Pests and Pathogens". Bee World. 98 (1): 2–6. doi:10.1080/0005772X.2020.1800330.
- Paulina Vidal, Alexandra Jones and Ursula Malone. "Emergency orders in place across NSW to protect bee industry from deadly varroa mite parasite". abc.net.au. ABC NEWS. Retrieved 26 June 2022.
- J. Raloff (August 8, 1998). "Russian queens bee-little mites' impact". Science News. Vol. 154, no. 6. p. 84.
- Frey, Eva; Rosenkranz, Peter (May 1, 2014). "Autumn invasion rates of Varroa destructor (Mesostigmata: Varroidae) into honey bee (Hymenoptera: Apidae) colonies and the resulting increase in mite populations". Journal of Economic Entomology. 107 (2): 508–515. doi:10.1603/EC13381. PMID 24772528. S2CID 25457602. Retrieved 1 August 2021.
- DeGrandi-Hoffman, Gloria; Curry, Robert (2004). "A mathematical model of Varroa mite (Varroa destructor Anderson and Trueman) and honeybee (Apis mellifera L.) population dynamics". International Journal of Acarology. 30 (4): 259–274. doi:10.1080/01647950408684393. S2CID 84974148. Retrieved 1 August 2021.
In the autumn though, brood rearing declines and the number of multiple-infested cells increases.
- Rosenkranz, Peter; Aumeier, Pia; Ziegelmann, Bettina (January 2010). "Biology and control of Varroa destructor". Journal of Invertebrate Pathology. 103: S96–S119. doi:10.1016/j.jip.2009.07.016. PMID 19909970.
- Peter G. Kevan; Terence M. Laverty & Harold A. Denmark (1990). "Association of Varroa jacobsoni with organisms other than honeybees and implications for its dispersal". Bee World. 71 (3): 119–121. doi:10.1080/0005772X.1990.11099048.
- "Varroosis : USDA ARS". www.ars.usda.gov. Archived from the original on March 12, 2023. Retrieved 27 October 2023.
- Potts, Simon G.; Biesmeijer, Jacobus C.; Kremen, Claire; Neumann, Peter; Schweiger, Oliver; Kunin, William E. (June 2010). "Global pollinator declines: trends, impacts and drivers". Trends in Ecology & Evolution. 25 (6): 345–353. doi:10.1016/j.tree.2010.01.007.
- Watanabe, Myrna E. (1 May 2008). "Colony Collapse Disorder: Many Suspects, No Smoking Gun". BioScience. 58 (5): 384–388. doi:10.1641/B580503.
- Le Conte, Yves; Ellis, Marion; Ritter, Wolfgang (May 2010). "Varroa mites and honey bee health: can Varroa explain part of the colony losses?". Apidologie. 41 (3): 353–363. doi:10.1051/apido/2010017.
- "Varroa mites: A step-by-step guide to monitoring in New York" (PDF). Pollinator Network at Cornell University.
- Jack, Cameron J; Ellis, James D (1 September 2021). "Integrated Pest Management Control of Varroa destructor (Acari: Varroidae), the Most Damaging Pest of ( Apis mellifera L. (Hymenoptera: Apidae)) Colonies". Journal of Insect Science. 21 (5). doi:10.1093/jisesa/ieab058. PMC 8449538.
- Milbrath, Meghan (January 2018). "Varroa Mite Monitoring: Using a Sugar Roll to Quantify Infestation of Varroa destructor in Honey Bee Colonies". Michigan Pollinator Initiative, Michigan State University.
- "Czech teacher battles bee-killing disease with hot hive". Reuters. 28 May 2017.
- Zotti, Moises; dos Santos, Ericmar Avila; Cagliari, Deise; Christiaens, Olivier; Taning, Clauvis Nji Tizi; Smagghe, Guy (June 2018). "RNA interference technology in crop protection against arthropod pests, pathogens and nematodes: RNA interference technology in crop protection against arthropod pests, pathogens and nematodes". Pest Management Science. 74 (6): 1239–1250. doi:10.1002/ps.4813.
- Victoria Gill (December 22, 2010). "Genetic weapon developed against honeybee-killer". BBC News. Retrieved February 24, 2011.
- Leclercq, Gil; Pannebakker, Bart; Gengler, Nicolas; Nguyen, Bach Kim; Francis, Frédéric (8 August 2017). "Drawbacks and benefits of hygienic behavior in honey bees ( Apis mellifera L.): a review". Journal of Apicultural Research. 56 (4): 366–375. doi:10.1080/00218839.2017.1327938.
- "A Sustainable Approach to Controlling Honey Bee Diseases and Varroa Mites". SARE. Retrieved 2008-11-18.
- Hunt, Greg; Given, J. Krispn; Tsuruda, Jennifer M.; Andino, Gladys K. (April 2016). "Breeding Mite-Biting Bees to Control Varroa" (PDF). Bee Culture.
Further reading
- Zhi-Qiang Zhang (2000). "Notes on Varroa destructor (Acari: Varroidae) parasitic on honeybees in New Zealand" (PDF). Systematic & Applied Acarology. Special Publications. 5: 9–14.
- Delaplane, Keith S (April 2015). "Varroa destructor: revolution in the making". Bee World. 82 (4): 157–159. doi:10.1080/0005772X.2001.11099522. S2CID 84549251.
- "Managing Varroa". Ministry of Agriculture, Fisheries and Food. 2005.
- Varroa Mite Controls Apiculture Factsheet #221, Ministry of Agriculture, Food and Fisheries, Government of British Columbia; April 2004
External links
- Varroa mite on the University of Florida / Institute of Food and Agricultural Sciences Featured Creatures website
- The ectoparasite mite Varroa destructor Anderson and Trueman in southeastern Brazil apiaries
- "Tiny mite responsible for bee colony deaths". CTV News. March 3, 2010.