Villin-1
Villin-1 is a 92.5 kDa tissue-specific actin-binding protein associated with the actin core bundle of the brush border.[1] Villin-1 is encoded by the VIL1 gene. Villin-1 contains multiple gelsolin-like domains capped by a small (8.5 kDa) "headpiece" at the C-terminus consisting of a fast and independently folding three-helix bundle that is stabilized by hydrophobic interactions.[2] The headpiece domain is a commonly studied protein in molecular dynamics due to its small size and fast folding kinetics and short primary sequence.[3][4]
| Villin-1 | |||||||
|---|---|---|---|---|---|---|---|
| Identifiers | |||||||
| Symbol | VIL1 | ||||||
| Alt. symbols | VIL | ||||||
| NCBI gene | 7429 | ||||||
| HGNC | 12690 | ||||||
| OMIM | 193040 | ||||||
| RefSeq | NM_007127 | ||||||
| UniProt | P09327 | ||||||
| Other data | |||||||
| Locus | Chr. 2 q35-q36 | ||||||
| |||||||
| villin 2 (ezrin) | |||||||
|---|---|---|---|---|---|---|---|
| Identifiers | |||||||
| Symbol | VIL2 | ||||||
| NCBI gene | 7430 | ||||||
| HGNC | 12691 | ||||||
| OMIM | 123900 | ||||||
| RefSeq | NM_003379 | ||||||
| UniProt | P15311 | ||||||
| Other data | |||||||
| Locus | Chr. 6 q22-q27 | ||||||
| |||||||
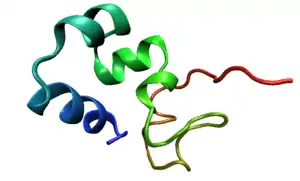
Structure
Villin-1 is made up of seven domains, six homologous domains make up the N-terminal core and the remaining domain makes up the C-terminal cap.[3] Villin contains three phosphatidylinositol 4,5-biphosphate (PIP2) binding sites, one of which is located at the head piece and the other two in the core.[5] The core domain is approximately 150 amino acid residues grouped in six repeats. On this core is an 87 residue, hydrophobic, C-terminal headpiece[1] The headpiece (HP67) is made up of a compact, 70 amino acid folded protein at the C-terminus. This headpiece contains an F-actin binding domain. Residues K38, E39, K65, 70-73:KKEK, G74, L75 and F76 surround a hydrophobic core and are believed to be involved in the binding of F-actin to villin-1. Residues E39 and K70 form a salt bridge buried within the headpiece which serves to connect N and C terminals. This salt bridge may also orient and fix the C-terminal residues involved in F-actin binding as in the absence of this salt bridge no binding occurs. A hydrophobic “cap” is formed by residue W64 side chains, which is completely conserved throughout the villin family. Below this cap is a crown of alternative positive and negative charged localities.[5] Villin can undergo post-translational modifications like tyrosine phosphorylation.[6] Villin-1 has the ability to dimerize and the dimerization site is located at the amino end of the protein.[7]
Expression
Villin-1 is an actin binding protein expressed mainly in the brush border of the epithelium in vertebrates but sometimes it is ubiquitously expressed in protists and plants.[4] Villin is found localized in the microvilli of the brush border of the epithelium lining of the gut and renal tubules in vertebrates.[5]
Function
Villin-1 is believed to function in the bundling, nucleation, capping and severing of actin filaments.[1] In vertebrates, villin proteins help to support the microfilaments of the microvilli of the brush border. However, knockout mice appear to show ultra-structurally normal microvilli reminding us that the function of villin is not definitively known; it may play a role in cell plasticity through F-actin severing.[5] The six-repeat villin core is responsible for Ca2+ actin severing while the headpiece is responsible for actin crosslinking and bundling (Ca independent). Villin is postulated to be the controlling protein for Ca2+ induced actin severing in the brush border. Ca2+ inhibits proteolytic cleavage of the domains of the 6 N-terminal core which inhibits actin severing.[3] In normal mice raising Ca2+ levels induces the severing of actin by villin, whereas in villin knockout mice this activity does not occur in response to heightened Ca2+ levels.[8] In the presence of low concentrations of Ca2+ the villin headpiece functions to bundle actin filaments whereas in the presence of high Ca2+ concentrations the N-terminal caps and severs these filaments.[1] The association of PIP2 with villin inhibits the actin capping and severing action and increases actin binding at the headpiece region, possibly through structural changes in the protein. PIP2 increases actin bundling not only by decreasing the severing action of villin but also through dissociating capping proteins, releasing actin monomers from sequestering proteins and stimulating actin nucleation and cross linking.[3]
Villin subdomain
The C-terminal subdomain of Villin Headpiece VHP67, denoted VHP35, is stabilised in part, by a buried cluster of three phenylalanine residues. Its small size and high helical content are expected to promote rapid folding, and this has been confirmed experimentally. Villin-4 C-terminal construct VHP76 in Arabidopsis thaliana has been shown to exhibit higher affinity for F-actin in increasing concentrations of Ca2+, which further confirms the function of villin.
Structure
It has a simple topology consisting of three α-helices that form a well-packed hydrophobic core.
Degradation and regulation
Currently, it is theorized the regulation of plant villins are caused by degradation via the binding protein auxin, which targets the headpiece domain (VHP).
See also
References
- Friederich E, Vancompernolle K, Louvard D, Vandekerckhove J (September 1999). "Villin function in the organization of the actin cytoskeleton. Correlation of in vivo effects to its biochemical activities in vitro". The Journal of Biological Chemistry. 274 (38): 26751–60. doi:10.1074/jbc.274.38.26751. PMID 10480879.
- Ghoshdastider U, Popp D, Burtnick LD, Robinson RC (November 2013). "The expanding superfamily of gelsolin homology domain proteins". Cytoskeleton. 70 (11): 775–95. doi:10.1002/cm.21149. PMID 24155256. S2CID 205643538.
- Bazari WL, Matsudaira P, Wallek M, Smeal T, Jakes R, Ahmed Y (July 1988). "Villin sequence and peptide map identify six homologous domains". Proceedings of the National Academy of Sciences of the United States of America. 85 (14): 4986–90. Bibcode:1988PNAS...85.4986B. doi:10.1073/pnas.85.14.4986. PMC 281672. PMID 2839826.
- Klahre U, Friederich E, Kost B, Louvard D, Chua NH (January 2000). "Villin-like actin-binding proteins are expressed ubiquitously in Arabidopsis". Plant Physiology. 122 (1): 35–48. doi:10.1104/pp.122.1.35. PMC 58842. PMID 10631247.
- Meng J, Vardar D, Wang Y, Guo HC, Head JF, McKnight CJ (September 2005). "High-resolution crystal structures of villin headpiece and mutants with reduced F-actin binding activity". Biochemistry. 44 (36): 11963–73. doi:10.1021/bi050850x. PMID 16142894.
- Panebra A, Ma SX, Zhai LW, Wang XT, Rhee SG, Khurana S (September 2001). "Regulation of phospholipase C-gamma(1) by the actin-regulatory protein villin". American Journal of Physiology. Cell Physiology. 281 (3): C1046-58. doi:10.1152/ajpcell.2001.281.3.C1046. PMID 11502583. S2CID 12089989.
- George SP, Wang Y, Mathew S, Srinivasan K, Khurana S (September 2007). "Dimerization and actin-bundling properties of villin and its role in the assembly of epithelial cell brush borders". The Journal of Biological Chemistry. 282 (36): 26528–41. doi:10.1074/jbc.M703617200. PMID 17606613.
- Revenu C, Courtois M, Michelot A, Sykes C, Louvard D, Robine S (2007). "Villin severing activity enhances actin-based motility in vivo". Molecular Biology of the Cell. 18 (3): 827–38. doi:10.1091/mbc.E06-05-0423. PMC 1805090. PMID 17182858.
Further reading
- "The Villin Family". The University of Edinburgh. 2000.
External links
- Villin at the U.S. National Library of Medicine Medical Subject Headings (MeSH)