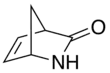
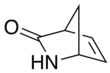
Vince lactam
Vince lactam[1] is the commercial name given to the bicyclic molecule γ-lactam 2-azabicyclo[2.2.1]hept-5-en-3-one. This lactam is a versatile chemical intermediate used in organic and medicinal chemistry. It is used as a synthetic precursor for three drugs (approved or in clinical trials).[2][3] It is named after Robert Vince who has used the structural features of this molecule for the preparation of carbocyclic nucleosides.[4] Vince's work with this lactam eventually led to his synthesis of abacavir.[5][6][7] Peramivir synthesis is also dependent on Vince lactam starting material.
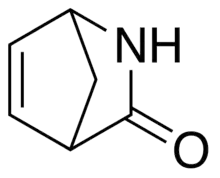 | |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
2-Azabicyclo[2.2.1]hept-5-en-3-one | |||
| Identifiers | |||
| |||
3D model (JSmol) |
| ||
| ChemSpider | |||
| EC Number |
| ||
PubChem CID |
|||
CompTox Dashboard (EPA) |
| ||
| |||
| |||
| Properties | |||
| C6H7NO | |||
| Molar mass | 109.128 g·mol−1 | ||
| Hazards | |||
| GHS labelling: | |||
  | |||
| Danger | |||
| H302, H317, H318 | |||
| P261, P264, P270, P272, P280, P301+P312, P302+P352, P305+P351+P338, P310, P321, P330, P333+P313, P363, P501 | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |||
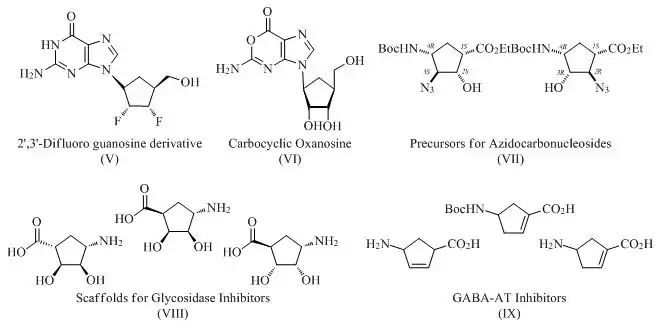
Vince lactam has been extensively used for the preparation of various carbocyclic nucleosides with medicinal applications in mind, including carbocyclic puromycin (I),[8] carbocyclic Ara-A (II),[9] carbovir (III)[10] and guanine as well as azaguanine carbocyclic derivatives (IV)[11]

Vince lactam is also an intermediate in the synthesis of various nucleoside analogs such as difluoro guanosine derivatives (V),[12][13] carbocyclic oxanosine and related derivatives (VI),[14] and precursors for azidocarbonucleosides (VII).[15] The lactam has found several applications in targeting an array of different diseased conditions by providing various non-nucleoside therapeutic molecules as well. Some well known examples include scaffolds for the preparation of glycosidase inhibitors (VIII)[16] and GABA-AT inhibitors (IX).[17]
References
- Singh, R.; Vince, R. Chem. Rev. 2012, 112 (8), pp 4642–4686."2-Azabicyclo[2.2.1]hept-5-en-3-one: Chemical Profile of a Versatile Synthetic Building Block and its Impact on the Development of Therapeutics"
- Rouhi, A. M. (July 14, 2003). "Simplifying Syntheses Is Always A Key Goal". C&EN. 80 (28): 40.
- Holt-Tiffin, K. E. Chimica Oggi 2009, 27, 23-25.
- "Robert Vince, Ph.D." Center for Drug Design, University of Minnesota.
- Daluge, S.; Vince, R. J. Org. Chem. 1978, 43, 2311-2320.
- Vince, R.; Hua, M. "Synthesis of carbovir and abacavir from a carbocyclic precursor" Current Protocols in nucleic acid chemistry Ed. Beaucage, S. L. 2006, Chapter 14 Unit 14.4. doi:10.1002/0471142700.nc1404s25.
- Vince, R. "A brief history of the development of Ziagen" Chemtracts 2008, 21, 127-134.
- Vince, R.; Daluge, S.; Brownell, J. J. Med. Chem. 1986, 29, 2400.
- Daluge, S.; Vince, R. J. Org. Chem., 1978, 43, 2311-2320.
- Vince, R.; Hua, M. J. Med. Chem. 1990, 33, 17.
- Peterson, M. L.; Vince, R. J. Med. Chem. 1990, 33, 1214-1219.
- Toyota, A.; Habutani, C.; Katagiri, N.; Kaneko, C. Tetrahedron Lett. 1994, 35, 5665-5668.
- Toyota, A.; Aizawa, M.; Habutani, C.; Katagiri, N.; Kaneko, C. Tetrahedron 1995, 36, 8783-8798.
- Saito, Y.; Nakamura, M.; Ohno, T.; Chaicharoenpong, C.; Ichikawa, E.; Yamamura, S.; Kato, K.; Umezawa, K. J. Antibiotics 2000, 53, 309-313.
- Kiss, L.; Forro, E.; Sillanpaa, R.; Fulop, F. Synthesis 2010, 153-160.
- Rommel, M.; Ernst, A.; Koert, U. Eur. J. Org. Chem. 2007, 4408-4430.
- Mineno, T.; Miller, M,. J. J. Org. Chem. 2003, 68, 6591-6596.