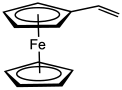
Vinylferrocene
Vinylferrocene is the organometallic compound with the formula (C5H5)Fe(C5H4CH=CH2). It is a derivative of ferrocene, with a vinyl group attached to one cyclopentadienyl ligand. As the ferrocene analogue of styrene, it is the precursor to some polyferrocenes.[1] It is an orange, air-stable oily solid that is soluble in nonpolar organic solvents.
 | |
| Names | |
|---|---|
| IUPAC name
Vinylferrocene | |
| Systematic IUPAC name
Ethenylferrocene | |
| Other names
Ferrocenylethene | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| |
| |
| Properties | |
| C12H12Fe | |
| Molar mass | 212.073 g·mol−1 |
| Appearance | orange solid |
| Melting point | 50–52 °C (122–126 °F; 323–325 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Vinylferrocene can be prepared by the dehydration of α-hydroxylethylferrocene, which is obtained from acetylferrocene.[2]
References
- Cass, Anthony E. G.; Davis, Graham; Francis, Graeme D.; Hill, H. Allen O.; Aston, William J.; Higgins, I. John; Plotkin, Elliot V.; Scott, Lesley D. L.; Turner, Anthony P. F. (1984). "Ferrocene-mediated enzyme electrode for amperometric determination of glucose". Analytical Chemistry. 56 (4): 667–671. doi:10.1021/ac00268a018. PMID 6721151.
- Rausch, Marvin D.; Siegel, Armand (1968). "Organometallic π-complexes. XIV. Vinylmetallocenes". Journal of Organometallic Chemistry. 11: 317–324. doi:10.1016/0022-328X(68)80054-3.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.