Vinyltriethoxysilane
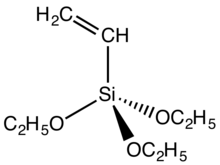
Vinyltriethoxysilane is an organosilicon compound with the formula (C2H5O)3SiCH=CH2. It is a colorless liquid. The compound is bifunctional, featuring both a vinyl group and hydrolytically sensitive ethoxysilyl groups. As such it is a crosslinking agent.[1]
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Ethenyltri(ethoxy)silane | |
| Other names
Triethoxyvinylsilane | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.000.984 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C8H18O3Si | |
| Molar mass | 190.31 |
| Appearance | Colorless liquid |
| Density | 0.903 g/cm3 |
| Boiling point | 160–161 °C (320–322 °F; 433–434 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Applications
Vinyltriethoxysilane and the related vinyltrimethoxysilane are used as monomers and comonomer for polymers such as ethylene-vinyltrimethoxysilane and ethylene-vinyl acetate-vinyltrimethoxysilane. Vinyltrialkoxysilanes are also used as cross-linking agents during the manufacture of cross-linked polyethylene (PEX). The alkoxysilane moiety is reactive toward water, and in the presence of moisture, it forms silicon-oxygen-silicon bonds that cross-link the material to cure it. Moisture-curable polymers are used as electrical insulation in some kinds of cables and for water pipe in under-floor heating installations.
Vinyltrialkoxysilanes are also used as a coupling agents or adhesion promoters for treatment of glass fibers and particulate minerals in order to form stronger bonds with resin and produce fiberglass with better mechanical properties. Amino-functional silanes such as (3-aminopropyl)triethoxysilane and epoxy-functional silanes are used for the same purpose. The silane group attaches to the glass substrate via covalent Si-O-Si bond, while the resin reacts with the vinyl-, amino-, or epoxy- group and binds to it.
References
- Wolff, Siegfried (1996). "Chemical Aspects of Rubber Reinforcement by Fillers". Rubber Chemistry and Technology. 69 (3): 325–346. doi:10.5254/1.3538376.