Vitamin A2
Vitamin A2 is a subcategory of vitamin A.[1]
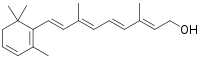 | |
| Names | |
|---|---|
| IUPAC name
3,4-Didehydroretinol | |
| Preferred IUPAC name
(2E,4E,6E,8E)-3,7-Dimethyl-9-(2,6,6-trimethylcyclohexa-1,3-dien-1-yl)nona-2,4,6,8-tetraen-1-ol | |
| Other names
Retinol 2; 3,4-Dehydroretinol | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.001.116 |
| EC Number |
|
| KEGG | |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C20H28O | |
| Molar mass | 284.443 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Dehydroretinal (3,4-dehydroretinal) belongs to the group of vitamin A2 as a retinaldehyde form, besides the endogenous 3,4-dehydroretinol (vitamin A2 alcohol), and 3,4-dehydroretinoic acid (vitamin A2 acid).[2][3]
Vitamin A2 was first identified by Richard Alan Morton using newly-developed absorption spectroscopy in 1941.[4]
References
- Babino D, Golczak M, Kiser PD, Wyss A, Placzewski K, von Lintig J (2016). "The Biochemical Basis of Vitamin A3 Production in Arthropod Vision". ACS Chem Biol. 11 (4): 1049–1057. doi:10.1021/acschembio.5b00967. PMC 4841470. PMID 26811964.
- Törmä H, Vahlquist A (1985). "Biosynthesis of 3-dehydroretinol (vitamin A2) from all-trans-retinol (vitamin A1) in human epidermis". J Invest Dermatol. 85 (6): 498–500. doi:10.1111/1523-1747.ep12277290. PMID 4067325.
- Vahlquist A (1980). "The identification of dehydroretinol (vitamin A2) in human skin". Experientia. 36 (3): 317–318. doi:10.1007/bf01952299. PMID 7371787. S2CID 31357743.
- Goodwin, T W (1977). "R. A. Morton". Nature. 266 (5600): 394. doi:10.1038/266394a0. S2CID 31211784. Retrieved 20 October 2022.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.