Volixibat
Volixibat (INN;[1] development code SHP626) is a medication under development as a possible treatment for nonalcoholic steatohepatitis (NASH), the most severe form of non-alcoholic fatty liver disease (NAFLD). No other pharmacotherapy yet exists for NASH, so there is interest in whether volixibat can prove to be both safe and effective. To encourage development and testing, the U.S. Food and Drug Administration (FDA) has issued fast track status.[2]
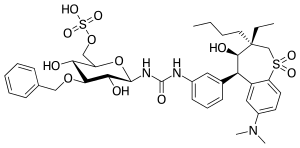 | |
| Clinical data | |
|---|---|
| Other names | N-(3-O-Benzyl-6-O-sulfo-β-D-glucopyranosyl)-N′-{3-[(3S,4R,5R)-3-butyl-7-(dimethylamino)-3-ethyl-4-hydroxy-1,1-dioxo-2,3,4,5-tetrahydro-1H-1λ6-benzothiepin-5-yl]phenyl}urea; SHP626 |
| Routes of administration | By mouth |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C38H51N3O12S2 |
| Molar mass | 805.96 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Volixibat is an IBAT inhibitor, meaning that it blocks the function of the IBAT protein (ileal bile acid transporter), which is also called SLC10A2 (solute carrier family 10 member 2) or ASBT (apical sodium–bile acid transporter). IBAT is most highly expressed in the ileum, where it is found on the brush border membrane of enterocytes. It is responsible for the initial uptake of bile acids, particularly conjugated bile acids, from the intestine as part of their enterohepatic circulation.[3]
References
- "International Nonproprietary Names for Pharmaceutical Substances (INN). Recommended International Nonproprietary Names: List 75" (PDF). World Health Organization. p. 166. Retrieved 21 January 2017.
- C, Deepak (August 3, 2016). "FDA Grants Fast Track Status to Volixibat". Internal Medicine News Digital Network. Frontline Medical Communications Inc., Parsippany, NJ, USA.
- Dawson PA (2011). "Role of the intestinal bile acid transporters in bile acid and drug disposition". Drug Transporters. Handbook of Experimental Pharmacology. Vol. 201. pp. 169–203. doi:10.1007/978-3-642-14541-4_4. ISBN 978-3-642-14540-7. PMC 3249407. PMID 21103970.