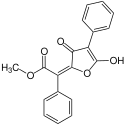
Vulpinic acid
Vulpinic acid is a natural product first found in and important in the symbiosis underlying the biology of lichens.[1] It is a simple methyl ester derivative of its parent compound, pulvinic acid, and a close relative of pulvinone, both of which derive from aromatic amino acids such as phenylalanine via secondary metabolism. The roles of vulpinic acid are not fully established, but may include properties that make it an antifeedant for herbivores. The compound is relatively toxic to mammals.
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Methyl (E)-(5-hydroxy-3-oxo-4-phenylfuran-2(3H)-ylidene)phenylacetate | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChEMBL | |||
| ECHA InfoCard | 100.007.560 | ||
PubChem CID |
|||
| UNII | |||
| |||
| Properties | |||
| C19H14O5 | |||
| Molar mass | 322.316 g·mol−1 | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards |
toxic | ||
| GHS labelling: | |||
 | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |||
Chemical description
Vulpinic acid was first isolated from lichens in 1925.[2] As an isolated, purified substance, it is bright yellow in color.[3]
Vulpinic acid is derived biosynthetically by esterification from pulvinic acid;[4] pulvinate itself derives from the aromatic amino acids phenylalanine and tyrosine, via dimerization and oxidative ring-cleavage of arylpyruvic acids, a process that also produces the related pulvinones.[5]
There have been several chemical syntheses reported for vulpinic acid. In one, butenolides were efficiently functionalized by Suzuki cross-coupling reactions via the corresponding enol triflates.[6]
Occurrence in lichens

Pulvinic acid is found in several lichen species, as well as some non-lichenized fungi.[2] It is a secondary metabolite of the fungal partner in the lichen symbiosis. It was found in the bolete fungus Pulveroboletus ravenelii.[7][5] In 2016, a new group of basidiomycetes distinct from the well known lichen fungal partner was implicated in producing vulpinic acid.[1]
Bioactivities
Vulpinic acid is relatively toxic to meat-eating mammals as well as insects and molluscs. However, it is not toxic to rabbits and mice. One biological function of vulpinic acid may be as a repellent that lichens have evolved to deter grazing by herbivores.[8] Lichens may also exploit the ultraviolet-blocking properties of the molecule, protecting the underlying photobionts.[9] For example, vulpinic acid is thought to function as a blue light screen in Letharia vulpina.[10] It had been shown previously to protect human skin cells in tissue culture against ultraviolet B-induced damage.[11]
Humans have exploited its mammalian toxicity, using lichens containing high amounts of the chemical (e.g., Letharia vulpina) to poison wolves in Scandinavia, sometimes adding it to baits containing reindeer blood and glass.[3]
Vulpinic acid has some antibacterial activity against gram-positive bacteria, and has been shown to disrupt cell division in MRSA.[12][13]
See also
References
- Spribille T, Tuovinen V, Resl P, Vanderpool D, Wolinski H, Aime MC, et al. (2016). "Basidiomycete yeasts in the cortex of ascomycete macrolichens". Science. 353 (6298): 488–492. Bibcode:2016Sci...353..488S. doi:10.1126/science.aaf8287. PMC 5793994. PMID 27445309.
- Mazza, Franc Paolo (1925). "Constitution and physical properties of vulpinic acid". Rendiconto dell'Accademia delle Scienze Napoli. 31: 182–190.
- Brodo, Irwin M.; Sharnoff, Sylvia Duran; Sharnoff, Stephen (2001). Lichens of North America. Yale University Press. p. 83. ISBN 978-0300082494.
- Crout, D.H.G. (2012). "The Biosynthesis of Carbocyclic Compounds". In Lloyd, D. (ed.). Carbocyclic Chemistry. Vol. One. Springer Science & Business Media. pp. 63–198, esp. 147. ISBN 9781468482706. Retrieved January 3, 2020.
- Gill, M.; Steglich, W. (1987). "Pigments of Fungi (Macromycetes)". Fortschritte der Chemie organischer Naturstoffe / Progress in the Chemistry of Organic Natural Products. pp. 1–297. doi:10.1007/978-3-7091-6971-1_1. ISBN 978-3-7091-7456-2. PMID 3315906.
{{cite book}}:|journal=ignored (help) - Ahmed, Zafar; Langer, Peter (2004). "Suzuki cross-coupling reactions of γ-alkylidenebutenolides: application to the synthesis of vulpinic acid". Journal of Organic Chemistry. 69 (11): 3753–3757. doi:10.1021/jo049780a. PMID 15153005.
- Duncan, Christine J.G.; Cuendet, Muriel; Fronczek, Frank R.; Pezzuto, John M.; Mehta, Rajendra G.; Hamann, Mark T.; Ross, Samir A. (2003). "Chemical and biological investigation of the fungus Pulveroboletus ravenelii". Journal of Natural Products. 66 (1): 103–107. doi:10.1021/np0203990. PMC 4969011. PMID 12542354.
- Nash, Thomas H. (1996). Lichen Biology. Cambridge University Press. p. 179. ISBN 978-0-521-45974-7.
- Legouin, Béatrice; Lohézic-Le Dévéhat, Françoise; Ferron, Solenn; Rouaud, Isabelle; Le Pogam, Pierre; Cornevin, Laurence; Bertrand, Michel; Boustie, Joël (2017). "Specialized metabolites of the lichen Vulpicida pinastri act as photoprotective agents". Molecules. 22 (7): 1162. doi:10.3390/molecules22071162. PMC 6152234. PMID 28704942.
- Phinney, Nathan H.; Gauslaa, Yngvar; Solhaug, Knut Asbjørn (2018). "Why chartreuse? The pigment vulpinic acid screens blue light in the lichen Letharia vulpina". Planta. 249 (3): 709–718. doi:10.1007/s00425-018-3034-3. PMID 30374913. S2CID 53102713.
- Varol, Mehmet; Türk, Ayşen; Candan, Mehmet; Tay, Turgay; Koparal, Ayşe Tansu (2016). "Photoprotective activity of vulpinic and gyrophoric acids toward ultraviolet B-induced damage in human keratinocytes". Phytotherapy Research. 30 (1): 9–15. doi:10.1002/ptr.5493. PMID 26463741. S2CID 206430748.
- Bačkor M, Hudá J, Repčák M, Ziegler W, Bačkorová M (1998). "The Influence of pH and Lichen Metabolites (Vulpinic Acid and (+)-Usnic Acid) on the Growth of the Lichen Photobiont Trebouxia irregularis". The Lichenologist. 30 (6): 577–582. doi:10.1017/S0024282992000574.
- Shrestha G, Thompson A, Robison R, St Clair LL (28 April 2015). "Letharia vulpina, a vulpinic acid containing lichen, targets cell membrane and cell division processes in methicillin-resistant Staphylococcus aureus". Pharmaceutical Biology. 54 (3): 413–8. doi:10.3109/13880209.2015.1038754. PMID 25919857. S2CID 37962126.