Waterborne resins
Waterborne resins are sometimes called water-based resins. They are resins or polymeric resins that use water as the carrying medium as opposed to solvent or solvent-less. Resins are used in the production of coatings, adhesives, sealants, elastomers and composite materials.[1][2] When the phrase waterborne resin is used, it usually describes all resins which have water as the main carrying solvent. The resin could be water-soluble,[3] water reducible or water dispersed.[4]
History
Most coatings have four basic components. These are the resin, solvent, pigment and additive systems[5] but the resin or binder is the key ingredient. Continuing environmental legislation in many countries along with geopolitics such as oil production are ensuring that chemists are increasingly turning to waterborne technology for paint/coatings and since resins or binders are the most important part of a coating, more of them are being developed and designed waterborne and there is a constantly increasing use by coating formulators. The use of waterborne coatings and hence waterborne resins really started to grow in the 1960s led by the United States and was driven by: a) the need to reduce flammability; b) environmental legislation aimed at reducing the amount of solvent vapor (VOC - Volatile organic compound) discharged into the atmosphere; c) cost; d) political factors i.e. security of supply.[6] All these factors helped the desire to reduce the reliance on oil derived solvents. The use of water as the carrying solvent for coatings and hence resins has been increasing ever since. The same holds true for adhesives. Water is generally a low cost (but not free) commodity in plentiful supply with no toxicity problems so there has always been a desire to produce paints, inks, adhesives and textile sizes etc. with water as the carrying solvent. This has required the production of waterborne resins designed for these systems. In recent years legislative pressure has ensured that waterborne systems and hence waterborne resins are coming increasingly to the fore.[7][8][9]
Types of waterborne resins
Waterborne epoxy resins
An epoxy resin system generally consists of a curing agent and an epoxy resin. Both the curing agent and the epoxy resin can be made waterborne. Solid epoxy resin (molecular weight >1000) dispersions are available and consist of an epoxy resin dispersed in water sometimes with the aid of co-solvents and surfactants. The resin backbone is often modified to ensure water dispersibility. These resins dry in their own right by water/co-solvent evaporation and the particles coalescence.[10] To cure the resin and crosslink it, an amine-based curing agent is usually added. This produces a two-component system. An alternative is to use standard medium viscosity liquid epoxy resins and emulsify them in a water-soluble polyamine or polyaminoamide hardener resin which also gives a two-component system. Polyaminoamides (or polyamidoamines) are made by reacting ethylene amines with dimerized fatty acids to give a species with amide links but still having amine functionality. Water is liberated during the condensation reaction. These resins can then be made water-soluble by reacting further with glacial organic acids or formaldehyde. Resins like these are usually left with yet further amine functionality on the polymer backbone to enable them to cure and crosslink an epoxy resin.[11] Paints may then be made from them by pigmenting either the epoxy or the amine hardener portion or even both.[12][13] Polyamine curing resins as opposed to polyaminoamide resins are generally made by partially adducting polyfunctional amines with an epoxy resin and/or epoxy diluent and leaving the species with residual amine functionality. This adduct can then be dissolved in water and used to emulsify more epoxy resin and again either portion or both may be pigmented. The advantage with these systems is that they do not need glacial organic acids to solubilize them. This is an advantage if the coating is to be used over a highly alkaline substrate such as fresh concrete, as the alkali from the cement will neutralise the acid and cause instability on repeated dipping of a brush into the can.[14] Even though water is present and is a fuel for corrosion, water-based metal coatings based on waterborne epoxy can also be formulated.[15] Other research is investigating the benefits of combining graphene technology with waterborne epoxy.[16]
Research continues and many patents and journal papers continue to be published with novel ways of converting epoxy systems to their waterborne counterparts. One such method is to take a molecule that already is intrinsically partially hydrophilic such as a diol with a polypropylene oxide backbone, and then reacting it with epichlorohydrin and then dehydrochlorinated with sodium hydroxide. This produces a diepoxy terminated polypropylene glycol molecule. This can now be reacted with an ethyleneamine such as triethylenetetramine (TETA) to produce an amine terminated moiety that is intrinsically hydrophilic and able to cure an epoxy resin.[17][18] These waterbased wpoxy coatings when used with the right choice of pigments, can be used to coat the inside of oil tanks.[19]
Waterborne alkyd resins
Water reducible alkyds are basically conventional alkyd resins (i.e., polyesters based on saturated or unsaturated oils or fatty acids, polybasic acids and alcohols) modified to confer water miscibility. Typical components are vegetable oils or fatty acids such as linseed, soyabean, castor, dehydrated castor, safflower, tung, coconut and tall oil. Acids include isophthalic, terephthalic, adipic, benzoic, succinic acids and phthalic, maleic and trimellitic anhydride. Polyols include glycerol, pentaerythritol, Trimethylolpropane, ethylene glycol, propylene glycol, diethylene glycol, neopentyl glycol, 1,6-hexanediol and 1,4-butanediol.[20] Typical methods for introducing varying degrees of water miscibility are similar to other resin systems. Methods basically involve introducing hydrophilic centres such as acid groups that can then be neutralised to form a salt.[21] Introducing polar groups onto the backbone is another method. With alkyds typical methods include maleinazation of unsaturated fatty acids with maleic anhydride. This involves making a Diels-Alder adduct near the double bond sites. The acid groups introduced can then be further reacted with polyols. A Diels-Alder reaction only occurs where there is a conjugated double bond system. Simple addition occurs if not conjugated. Other techniques include synthesizing the resin with hydroxyl functional oligomers e.g. containing ethylene glycol then adding specific acid or hydroxyl containing substances towards the end of the reaction. Another technique is making an acrylic functional alkyd with an acrylic monomer blend rich in carboxylic acid groups.
Alkyd emulsions
Late twentieth century technology allowed the production of alkyd emulsions.[22] The technology continues to evolve including production of DTM (Direct To Metal) finishes.[23] The biggest issue has been getting VOC content below 250g/L. Poor corrosion resistance has also been an issue. Alkyd emulsion technology uses a reactive surfactant that has double bonds and thus oxidative drying properties like a conventional alkyd. The material is then put under shear and water added slowly. Initially a water in oil emulsion is formed but continued water addition and shear results in inversion and a stable oil in water emulsion is formed.[24][25] Sustainability and other market factors mean a number of companies are entering the market.[26] As well as patents, doctoral theses are being done at universities on the subject.[27]
Waterborne polyester resins
Saturated polyester resins contain many of the materials used in conventional alkyd resins but without the oil or fatty acid components. Typical components for these resins are poly carboxylic and polyhydroxyl components. The more commonly used polyacids are phthalic, isophthalic, terephthalic and adipic acid. Phthalic and trimellitic anhydrides may also be used. Polyols tend to be neopentyl glycol, 1,6-hexanediol and trimethylolpropane. To make them waterborne organic acids or anhydrides are added in a two-stage process but there are other methods too.[28][29]
Waterborne polyurethane resins
Polyurethanes resins are available waterborne. The single component versions are usually referred to as Polyurethane dispersions (PUD). They are available in anionic, cationic and nonionic versions though anionic moieties are the most readily available commercially.[30] The use of an anionic or cationic center or indeed a hydrophilic non-ionic manufacturing technique tends to result in a permanent inbuilt water resistance weakness. Research is being conducted and techniques developed to combat this weakness.[31] Cationic PUD also introduce hydrophilic components when synthesized, but techniques have and are being researched to improve the performance and water resistance properties by various techniques. This includes introducing star-branched polydimethylsiloxane.[32]
Waterborne polyurethanes are also available in 2 component versions.[33] As a 2 component polyurethane consists of polyol(s) and an isocyanate and isocyanates react with water this requires special formulating and production techniques.[34][35] The polyisocyanate that is water-dispersible maybe modified with sulfonate[36] for example. PUDs are not usually synthesised with plant based polyols because they don't have other performance enhancing functional groups. Recent work (2021) reports modification to achieve this and enable even greener versions.[37] Work is also ongoing to get the performance of 1 component waterborne polyurethanes to match that of 2 component versions.[38] Self-healing versions of two-component waterborne polyurethanes are being researched.[39] Research has shown that modification of these resin systems with polyaniline improves a number of properties including corrosion resistance.[40]
Ionic centers are usually introduced with waterborne PUDs, and so the water resistance in the resultant film has been studied. The nature of the polyol and the level of COOH groups and hydrophobic modification with other moieties can improve the hydrophilicity. Polyester polyols give the biggest improvements.[41][42] Polycarbonate polyols also enhance properties,[43] especially if the polycarbonate is also fluorinated.[44]
Silicone modification of the resin makes the species much more hydrophobic and water resistant.[45][46][47]
As the world attempts to move towards a low-carbon economy, carbon capture by using carbon dioxide from the atmosphere is gaining attention and research being done. Using carbon dioxide in PUD production is being researched.[48]
Waterborne lattices
A latex is a stable dispersion (emulsion) of polymer in water. Synthetic lattices are usually made by polymerizing a monomer such as vinyl acetate that has been emulsified with surfactants dispersed in water.[49] The overall technique is called Emulsion polymerization. Other techniques including inversion from water in oil to oil in water emulsions are available.[50] Particular emphasis in recent years has been the production of self-crosslinking versions especially acrylic emulsions. As an example, these may be produced by modifying with divinyl silane.[51] Some examples include vinyl acetate based latices, acrylics and styrene-butadiene versions. They may be used to produce waterborne direct to metal coatings.[52] Waterborne acrylic resins are also used frequently in water-based paints.[53]
Acrylic latices prepared by emulsion polymerization are often improved by copolymerizing other functional monomers.[54] Glycidyl methacrylate is one such monomer used which then incorporates oxirane functionality into the polymer. This would then improve the properties (such as scrub resistance) of the paint formulated from this resin. DMAEMA (dimethylaminoethyl methacrylate) is another such species.[55] Other innovative techniques for improving acrylic latices include incorporating a biocide with acrylic functionality as the modifying monomer. This allows the binder for a waterborne paint to be inherently anti-biocidal.[56] Techniques exist to speed up the cure of waterborne acrylics.[57] Waterborne acrylic latices and polyurethane acrylates that are UV curable have also been produced.[58]
Polymeric and oligomeric aziridines are one of the moieties used to crosslink waterborne resins. They usually react with the carboxyl groups present on these species. Potlife is usually improved along with other properties.[59]
Emulsion polymerization: Polymerization whereby monomer(s), initiator, dispersion
medium, and possibly colloid stabilizer constitute initially an inhomogeneous system
resulting in particles of colloidal dimensions containing the formed polymer.Note: With the exception of mini-emulsion polymerization, the term “emulsion polymerization”
does not mean that polymerization occurs in the droplets of a monomer emulsion.[60]Batch emulsion polymerization: Emulsion polymerization in which all the ingredients are
placed in a reactor prior to reaction.[61]
Waterborne electrophoretic deposition resins
see article Electrophoretic deposition
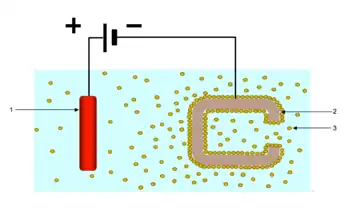
The resins used for electrodeposition are usually epoxy, acrylic or phenolic resin types. They are formulated with functional groups which when neutralised form ionic groups on the polymer backbone. These confer water solubility on the polymer. They are available as anodic versions which deposit on the cathode of an electrochemical cell or cathodic which deposit on the cathode.[62] Cathodic electrodeposition resins dominate and they have revolutionised corrosion protection in the automotive industry. Ceramics as well as metals may be coated this way.[63] They are applied as OEM (Original Equipment Manufacture) rather than as a refinishing system. Cathodic resins contain amines on the polymer backbone which are neutralized by acids groups such as acetic acid to give a stable aqueous dispersion. When an electric current is passed through a car body that is dipped in a bath containing a paint based on a cathodic electrodeposition resin, the hydroxyl ions formed near the cathode deposit the paint on the car body. The electric current needed for this is determined by the number of ionic centers. Dispersions of waterborne resins for electrocoating usually contain some co-solvents such as butyl glycol and isopropanol and are usually very low in solids content i.e. 15%. They usually have molecular weights in the region of 3000–4000. Paints based on them tend to have PVCs of less than 10 i.e. a very high binder to pigment ratio.
Cathodic electrophoretic deposition coatings can be made that are self-healing even at room temperature. The base polymer used for this synthesis is, a waterborne Polyurethane Dispersion (PUD) that is cationic rather than anionic.[64]
Waterborne hybrid resins
Many resins are available waterborne but can be hybrids or blends. An example would be polyurethane dispersions blended or hybridized with acrylic resins,[65][66] which are commonly used in automotive paint. Such systems can be made by using acrylic monomers and a polyurethane dispersion which will polymerise simultaneously to give an interpenetrating polymer network, without the need for NMP as a cosolvent. This combines the lower cost of acrylic with the high performance of a polyurethane.[67] Waterborne epoxy resins may be modified with acrylate and then further modified with side chains having many fluorine atoms on them.[68] Waterborne resins are also available that use both water and renewable raw materials.[69] Another example is to combine alkyd resins with acrylics to make them waterborne. Using hyperbranched alkyds and modifying them with acrylic monomers and using mini emulsion polymerization, suitable hybrids maybe formed.[70] As well as hybridization of the resins, a combination of techniques maybe employed. As an example, ultraviolet curing coatings that can be electrodeposited and are waterborne hybrids of epoxy and acrylic resins maybe produced.[71][72]
Hybrid resins include among others, PUDs that are both waterborne and UV curable. They are being researched and many papers published.[73][74][75][76][77][78] PUD- acrylics using epoxidized soybean oil have been produced that are UV curable.[79] The structure and type of acrylate will affect the properties.[80] Hybrid resins used in coatings that are vegetable based, waterborne and UV curable are considered very green and have also been investigated.[81][82] Similarly, UV-curable waterborne fluorinated polyurethane-acrylate resins can be designed and used in coatings.[83] As well as acrylic PUD hybridization, further modification with silane monomers can be undertaken.[84]
Other examples of hybridization include modifying waterborne epoxy with latex dispersions. The latex-modified epoxy aqueous dispersions are treated by evaporation techniques. Nitrile latices were used in the study.[85]
Modification of soybean oil that has been epoxidized and then reacted with acrylic acid will produce waterborne epoxy acrylates that are also based on some renewable content. The corrosion resistance properties are improved using this technique.[86]
Alkyds can likewise be hybridised and made water reducible. This may be achieved by acrylic modification.[87] Waterborne epoxy resins may also be acrylated and hybridized and much research has gone into these systems.[88][89][90][91]
Waterborne resins with high bio-based or renewable content
High bio-based content or renewability of materials is highly prized as there is a trend in some parts of the world to a low-carbon economy.[92] Waterborne resins are already perceived as environmentally friendly but work is ongoing to improve this further by using non-petroleum based raw materials where possible.[93] Waterborne epoxies are one such area of research.[94]
Water

Water is in some ways an unusual chemical. It is a very powerful and universal solvent. Most liquids reduce in volume on freezing, but water expands. It occurs naturally on earth in all three states of solid (ice), liquid (water) and gas(water vapour and steam). At 273.16 K or 0.16 °C (known as the triple point) it can coexist in all three states simultaneously. It has a very low molecular weight of 18 and yet a relatively high boiling point of 100 0 C. This is due to inter molecular forces and in particular hydrogen bonding. The surface tension is also high at 72 dynes/cm (mN/metre) which affects its ability to wet certain surfaces. It evaporates (latent heat of evaporation 2260 kJ per kg) very slowly in comparison to some solvents and hardly at all when the relative humidity is very high. It has a very high specific heat capacity (4.184 kJ/kg/K ) and that is why it is used in central heating systems in the United Kingdom and Europe. These factors have to be borne in mind when formulating waterborne resins and other water based systems such as adhesives and coatings.[95][96]
Uses
Waterborne resins find use in Coatings, Adhesives, Sealants and Elastomers and other applications. Specifically they find use in industrial coatings,[97] UV coatings,[98] floor coatings,[99] hygiene coatings,[100] wood coatings,[101] adhesives,[102] concrete coatings,[103] automotive coatings,[104][105] clear coatings[106] and anticorrosive applications including waterborne epoxy based anticorrosive primers[107][108][109] They are also used in the design and manufacture of medical devices such as the polyurethane dressing, a liquid bandage based on polyurethane dispersion.[110] Over the years they have also been used in polymer modified cements and repair mortars[111] They have also found use in general textile applications including coating nonwovens.[112]
References
- Padget, John (1994). "Polymers for waterbased coatings - A Systematic Overview". JCT Journal of Coatings Technology. 66 (839): 89–105.
- Annable, T; Brown, R A; Padget, J C; van den Elshout, A (July 1998). "Improvements in the application properties of water-based low VOC coatings". Surface Coatings International. 81 (7): 321–329. doi:10.1007/bf02700556. ISSN 1356-0751. S2CID 59152908.
- Bicak, Niyazi; Gazi, Mustafa; Karagoz, Bunyamin (2006-01-01). "New water-soluble polymer with allyl pendant groups". Designed Monomers and Polymers. 9 (2): 193–200. doi:10.1163/156855506776382646. S2CID 101639021.
- "Waterborne Resins". Allnex. Archived from the original on 2020-03-25. Retrieved 2020-03-24.
- Waterborne coatings and additives. Karsa, D. R., Davies, W. D., Royal Society of Chemistry (Great Britain), Society of Chemical Industry (Great Britain). Cambridge, UK: Royal Society of Chemistry. 1995. ISBN 0-85404-740-9. OCLC 33164476.
{{cite book}}: CS1 maint: others (link) - Jackson, K. (1999-07-01). "Recent advances in water-borne protective coatings". Surface Coatings International. 82 (7): 340–343. doi:10.1007/BF02720130. ISSN 1356-0751. S2CID 135613088.
- Thames S.F. “Conversion to Water-Soluble/Water-borne polymers” February 1996 Lecture notes from the University of Southern Mississippi short course
- "Waterborne Industrial Coatings: Evolving Perceptions and Technologies". www.coatingstech-digital.org. Archived from the original on 2022-01-28. Retrieved 2021-07-22.
- "CoatingsTech - Waterborne Direct-to-Metal Coatings: Enduring Solutions in Corrosion Protection". www.coatingstech-digital.org. Retrieved 2022-07-07.
- Darwen S. “Developments in high performance water-borne epoxy coatings” Polymers Paints and Colours Journal February. 23 1994 pages 65–67
- Richardson F.B “Water-borne Epoxy Coatings: Past, Present and Future” Modern Paints and Coatings April 1988 pages 84-88
- Howarth, Graham (1995-01-01). "The use of water‐based epoxies for anti‐corrosive primers". Pigment & Resin Technology. 24 (6): 3–6. doi:10.1108/eb043156. ISSN 0369-9420.
- Ranjbar, Zahra (2009-01-01). "Optimization of a Waterborne Epoxy Coatings Formulation via Experimental Design".
{{cite journal}}: Cite journal requires|journal=(help) - Howarth G.A "Synthesis of a legislation compliant corrosion protection coating system based on urethane, oxazolidine and waterborne epoxy technology" Master of Science Thesis April 1997 Imperial College London
- "Formulate Waterborne Metal Epoxy Coatings That Work". coatings.specialchem.com. Archived from the original on 2021-01-14. Retrieved 2021-01-13.
- "Waterborne Epoxy Technology:Lifting Performance for Tomorrow". CoatingsTech February 2022: 35–38. Retrieved 2022-02-18.
- Huang, Wang, Lai, Li, Jiang & Zhang (March 2021). "Fabrication of a nonionic self-emulsifiable waterborne epoxy curing agent with high cure properties". Journal of Coatings Technology & Research. 18 (2): 549–558. doi:10.1007/s11998-020-00423-3. S2CID 230717990.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - US 5596030, Walker, Frederick H., "Amide-containing self-emulsifying epoxy curing agent", published 1997-01-21, assigned to Air Products & Chemicals Inc.
- Chen, Zhong-Hua; Tang, Ying; Yu, Fei; Chen, Jian-Hua; Chen, Hai-Hong (2008-06-01). "Preparation of light color antistatic and anticorrosive waterborne epoxy coating for oil tanks". Journal of Coatings Technology and Research. 5 (2): 259–269. doi:10.1007/s11998-007-9063-7. ISSN 1935-3804. S2CID 137473922. Archived from the original on 2023-03-14. Retrieved 2023-03-14.
- US 5137965, Knox, David E., "Water-borne alkyd resin compositions", published 1992-08-11, assigned to Westvaco Corp.
- "Synthesis of waterborne alkyd resins". Archived from the original on 2022-01-28. Retrieved 2020-03-24.
- Östberg, G.; Huldén, M.; Bergenståhl, B.; Holmberg, K. (1994-06-01). "Alkyd emulsions". Progress in Organic Coatings. 24 (1–4): 281–297. doi:10.1016/0033-0655(94)85020-8. ISSN 0300-9440. Archived from the original on 2022-01-28. Retrieved 2021-05-24.
- "Fast-Dry DTM Alkyd Emulsion". www.pcimag.com. Archived from the original on 2021-04-26. Retrieved 2021-04-26.
- Asian Paints (2018-05-24). "(WO2018092158) Water Borne Alkyd Emulsions for Surface Primer Compositions for architectural interior finishes". Paints and Coatings Expert. Archived from the original on 2021-04-26. Retrieved 2021-04-26.
- "RX Series Alkyd Emulsifiers". Ethox. Archived from the original on 2021-04-26. Retrieved 2021-04-26.
- "Arkema Expands Manufacturing Of Alkyd Emulsions For Sustainable Formulating". Coatings World. Archived from the original on 2021-04-26. Retrieved 2021-04-26.
- Burns, Molly Elise (August 2016). "A comparison of solvent and water-borne Alkyd Coatings & The History of VOC Regulation in the United States Master of Science Thesis". Faculty of California Polytechnic State University. Archived from the original on 2021-04-25. Retrieved 2021-04-26.
- US 5218042, Kuo, Thauming & Moody, Keith M., "title=Water-dispersible polyester resins and process for their preparation", published 1993-06-08
- US 8309229, Nakahara, Shuichi & Harakawa, Hiromi, "Polyester resin and thermosetting water-borne coating compositions", published 2012-11-13, assigned to Kansai Paint Co. Ltd.
- "Water Based Polyurethanes Dispersions(PUDs)-An Overview". www.linkedin.com. Archived from the original on 2018-11-10. Retrieved 2020-03-24.
- Xu, Liangfeng (July 2021). "CO2 triggered hydrophobic/hydrophilic switchable waterborne polyurethane-acrylate with simultaneously improved water resistance and mechanical properties". Journal of Coatings Technology and Research. American Coatings Association. 18 (4): 989–998. doi:10.1007/s11998-021-00476-y. ISSN 1547-0091. S2CID 233176697.
- He, Xiaoling; He, Jingwei; Sun, Yangkun; Zhou, Xiaopei; Zhang, Jingying; Liu, Fang (2022-07-01). "Preparation and characterization of cationic waterborne polyurethanes containing a star-branched polydimethylsiloxane". Journal of Coatings Technology and Research. 19 (4): 1055–1066. doi:10.1007/s11998-021-00584-9. ISSN 1935-3804. S2CID 246946432.
- Wang, Li; Xu, Fei; Li, Hongxin; Liu, Yangyan; Liu, Yali (2017-01-01). "Preparation and stability of aqueous acrylic polyol dispersions for two-component waterborne polyurethane". Journal of Coatings Technology and Research. 14 (1): 215–223. doi:10.1007/s11998-016-9845-x. ISSN 1935-3804. S2CID 100045366. Archived from the original on 2023-03-14. Retrieved 2023-02-20.
- EP 2523987, Nachshon-Galili, Nitsa & Sussan, Reut, "Two-component water-based polyurethane compositions and coatings", published 2012-11-21, assigned to Pazkar Ltd.
- "The Case for Two-Component Waterborne Polyurethane Coatings". www.pcimag.com. Archived from the original on 2020-03-25. Retrieved 2020-03-25.
- Peng, Zhongkang (2020). "Synthesis and properties of water-dispersible polyisocyanates carrying sulfonate". J. Coat. Technol. Res. 17 (2): 345–359. doi:10.1007/s11998-019-00277-4. S2CID 207989601.
- "Biobased Polyol with self-crosslinking functionality". www.coatingstech-digital.org. p. 32. Archived from the original on 2022-01-28. Retrieved 2022-01-20.
- "1K PUR Dispersion with Comparable Performance to 2K Waterborne Coating". CoatingsTech February 2022. Retrieved 2022-02-18.
- Xu, Xinmeng; Zhou, Zhongqun; Qin, Liangrong; Yu, Caili; Zhang, Faai (2022-05-01). "Preparation of PVA/PU/PUA microcapsules and application in self-healing two-component waterborne polyurethane coatings". Journal of Coatings Technology and Research. 19 (3): 977–988. doi:10.1007/s11998-021-00577-8. ISSN 1935-3804. S2CID 246752459.
- Li, Xianwen; Xu, Xinmeng; Zhang, Faai (2023-05-01). "Antistatic and antibacterial two-component waterborne polyurethane coating". Journal of Coatings Technology and Research. 20 (3): 869–881. doi:10.1007/s11998-022-00708-9. ISSN 1935-3804. S2CID 254625069.
- Song, Sam Cha; Kim, Suk Joon; Park, Kyung-Kyu; Oh, Joong-Geul; Bae, Seong-Guk; Noh, Geon Ho; Lee, Won-Ki (2017-12-12). "Synthesis and properties of waterborne UV-curable polyurethane acrylates using functional isocyanate". Molecular Crystals and Liquid Crystals. 659 (1): 40–45. doi:10.1080/15421406.2018.1450824. ISSN 1542-1406. S2CID 102697178.
- Bai, Chen Yan; Zhang, Xing Yuan; Dai, Jia Bing; Zhang, Chu Yin (2007-07-02). "Water resistance of the membranes for UV curable waterborne polyurethane dispersions". Progress in Organic Coatings. 59 (4): 331–336. doi:10.1016/j.porgcoat.2007.05.003. ISSN 0300-9440.
- Hwang, Hyeon-Deuk; Park, Cho-Hee; Moon, Je-Ik; Kim, Hyun-Joong; Masubuchi, Tetsuo (2011-12-01). "UV-curing behavior and physical properties of waterborne UV-curable polycarbonate-based polyurethane dispersion". Progress in Organic Coatings. 72 (4): 663–675. doi:10.1016/j.porgcoat.2011.07.009. ISSN 0300-9440.
- Hwang, Hyeon-Deuk; Kim, Hyun-Joong (2011-10-15). "UV-curable low surface energy fluorinated polycarbonate-based polyurethane dispersion". Journal of Colloid and Interface Science. 362 (2): 274–284. Bibcode:2011JCIS..362..274H. doi:10.1016/j.jcis.2011.06.044. ISSN 0021-9797. PMID 21788027.
- Zhang, Dinglun; Liu, Jin; Li, Zhen; Shen, Yun; Wang, Ping; Wang, Di; Wang, Xianbiao; Hu, Xianhai (2021-11-01). "Preparation and properties of UV-curable waterborne silicon-containing polyurethane acrylate emulsion". Progress in Organic Coatings. 160: 106503. doi:10.1016/j.porgcoat.2021.106503. ISSN 0300-9440. S2CID 240504048.
- Bai, Chenyan; Zhang, Xingyuan; Dai, Jiabing (2007-08-01). "Synthesis and characterization of PDMS modified UV-curable waterborne polyurethane dispersions for soft tact layers". Progress in Organic Coatings. 60 (1): 63–68. doi:10.1016/j.porgcoat.2007.07.003. ISSN 0300-9440.
- Hong, Chengqi; Zhou, Xing; Ye, Yuanchao; Li, Wenbo (2021-07-01). "Synthesis and characterization of UV-curable waterborne Polyurethane–acrylate modified with hydroxyl-terminated polydimethylsiloxane: UV-cured film with excellent water resistance". Progress in Organic Coatings. 156: 106251. doi:10.1016/j.porgcoat.2021.106251. ISSN 0300-9440. S2CID 233549036.
- Wang, Jin; Zhang, Hongming; Miao, Yuyang; Qiao, Lijun; Wang, Xianhong; Wang, Fosong (2016-09-25). "UV-curable waterborne polyurethane from CO2-polyol with high hydrolysis resistance". Polymer. 100: 219–226. doi:10.1016/j.polymer.2016.08.039. ISSN 0032-3861.
- "Definition of LATEX". www.merriam-webster.com. Archived from the original on 2020-03-25. Retrieved 2020-03-25.
- Bartoň, Jaroslav; Sarov, Yanko; Capek, Ignác (2006-01-01). "Polymerization of vinyl monomers in separated Winsor II (w/o) and Winsor I (o/w) microemulsion phases. Part 1: preparation and characterization of polymerizable vinyl-monomer-containing microemulsions". Designed Monomers and Polymers. 9 (2): 153–168. doi:10.1163/156855506776382682. S2CID 98198031.
- Chen, Xiaolong; Cao, Shusen; Zhao, Wangting; Chen, Lijun (2022-05-01). "Preparation and characterization of self-crosslinking acrylate emulsion modified by divinyl silane". Journal of Coatings Technology and Research. 19 (3): 887–895. doi:10.1007/s11998-021-00566-x. ISSN 1935-3804. S2CID 245964323.
- "Waterborne Direct to metal coatings:Enduring solutions in corrosion protection". www.coatingstech-digital.org. Retrieved 2022-07-21.
- Stoye, D.; Funke, W.; Hoppe, L.; et al. (2006). "Paints and Coatings". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a18_359.pub2.
- Geurts, J.; Bouman, J.; Overbeek, A. (2008-03-01). "New waterborne acrylic binders for zero VOC paints". Journal of Coatings Technology and Research. 5 (1): 57–63. doi:10.1007/s11998-007-9036-x. ISSN 1935-3804. S2CID 137589975. Retrieved 2023-03-14.
- Akbulut, Gokhan; Bulbul Sonmez, Hayal (2022-09-01). "Synthesis of styrene and n-butyl acrylate latex polymers modified by functional monomers and their waterborne paint applications". Journal of Coatings Technology and Research. 19 (5): 1421–1435. doi:10.1007/s11998-022-00616-y. ISSN 1935-3804. S2CID 248837079.
- Zhou, Xiaopei; Liu, Fang; Xiong, Shaobo; Zhou, Furong; Xiang, Hui; He, Jingwei (2022-09-01). "Preparation and properties of antibacterial styrene-acrylic emulsion containing thiazole structure and its application as coating". Journal of Coatings Technology and Research. 19 (5): 1365–1379. doi:10.1007/s11998-021-00589-4. ISSN 1935-3804. S2CID 249650102.
- Decker, C.; Masson, F.; Schwalm, R. (2004-04-01). "How to speed up the UV curing of water-based acrylic coatings". JCT Research. 1 (2): 127–136. doi:10.1007/s11998-004-0007-1. ISSN 1935-3804. S2CID 97686666.
- Decker, C.; Lorinczova, I. (2004-10-01). "UV-Radiation curing of waterborne acrylate coatings". JCT Research. 1 (4): 247–256. doi:10.1007/s11998-004-0027-x. ISSN 1935-3804. S2CID 95089921.
- Bückmann, A. J. P.; Chen, Q.; Overbeek, G. C.; Stals, P. J. M.; van der Zwaag, D. (2022-09-01). "Polymeric aziridines as benign crosslinkers for water-based coating applications". Journal of Coatings Technology and Research. 19 (5): 1345–1355. doi:10.1007/s11998-022-00626-w. ISSN 1935-3804. S2CID 250181669.
- Slomkowski, Stanislaw; Alemán, José V.; Gilbert, Robert G.; Hess, Michael; Horie, Kazuyuki; Jones, Richard G.; Kubisa, Przemyslaw; Meisel, Ingrid; Mormann, Werner; Penczek, Stanisław; Stepto, Robert F. T. (2011). "Terminology of polymers and polymerization processes in dispersed systems (IUPAC Recommendations 2011)" (PDF). Pure and Applied Chemistry. 83 (12): 2229–2259. doi:10.1351/PAC-REC-10-06-03. S2CID 96812603. Archived from the original (PDF) on 2013-10-20. Retrieved 2022-07-21.
- Slomkowski, Stanislaw; Alemán, José V.; Gilbert, Robert G.; Hess, Michael; Horie, Kazuyuki; Jones, Richard G.; Kubisa, Przemyslaw; Meisel, Ingrid; Mormann, Werner; Penczek, Stanisław; Stepto, Robert F. T. (2011). "Terminology of polymers and polymerization processes in dispersed systems (IUPAC Recommendations 2011)" (PDF). Pure and Applied Chemistry. 83 (12): 2229–2259. doi:10.1351/PAC-REC-10-06-03. S2CID 96812603. Archived from the original (PDF) on 2013-10-20. Retrieved 2022-07-21.
- Sato, Toshihiko (1982-01-01). "Mechanism of Electrophoretic Deposition of Organic Coatings on Anodized Aluminium". Transactions of the IMF. 60 (1): 25–30. doi:10.1080/00202967.1982.11870598. ISSN 0020-2967.
- Zhitomirsky, I. (2002-03-29). "Cathodic electrodeposition of ceramic and organoceramic materials. Fundamental aspects". Advances in Colloid and Interface Science. 97 (1): 279–317. doi:10.1016/S0001-8686(01)00068-9. ISSN 0001-8686. PMID 12027023.
- Li, Yingyu; He, Jingwei; Luo, Hongfeng; He, Xiaoling; Liu, Fang (2022-09-01). "Synthesis and property of room-temperature self-healable cathodic electrophoretic deposition coatings based on cationic waterborne polyurethane". Journal of Coatings Technology and Research. 19 (5): 1621–1633. doi:10.1007/s11998-022-00634-w. ISSN 1935-3804. S2CID 250120281.
- Tyre, C. Ivan (May 2008). "Utilization of Polyurethane-Acrylic blends to achieve optimum performance in 1K Water-Based Wood floor coating" (PDF). American Coatings Association. pp. 60–64.
- "BASF Insights | Where business meets science". insights.basf.com. Archived from the original on 2020-03-31. Retrieved 2020-03-31.
- "Coatings World - April 2022 - page34". coatingsworld.texterity.com. Retrieved 2022-04-19.
- Shi, Hongyi (2020). "Waterborne epoxy resins modified by reactive polyacrylate modifier with fluorinated side chains". J. Coat. Technol. Res. 17 (2): 427–437. doi:10.1007/s11998-019-00288-1. S2CID 209392485.
- "CoatingsTech - May 2020 - page20". www.coatingstech-digital.org. Archived from the original on 2022-01-28. Retrieved 2020-05-14.
- Murillo, Percino and Lopez (September 2019). "Colloidal, morphological, thermal, rheological and film properties of waterborne hyperbranched alkyd-acrylic resins". Journal of Coatings Technology and Research. 16 (5): 1223–1232. doi:10.1007/s11998-019-00205-6. S2CID 149769647.
- Chen, Yuan, Tu, Peng, Hu and Wang (September 2019). "Electrophoretic deposition of waterborne ultraviolet (UV)-curable coatings based on microgels". Journal of Coatings Technology and Research. 16 (5): 1367–1378. doi:10.1007/s11998-019-00219-0. S2CID 189973324.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Shi, Liu, Liu, He, Jang and Wang (September 2019). "UV-curable waterborne epoxy acrylate coating modified by monomethacryloyloxy-terminated fluorinated oligomer". Journal of Coatings Technology and Research. 16 (5): 1305–1316. doi:10.1007/s11998-019-00209-2. S2CID 150036770.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Yang, Zhenglong; Wicks, Douglas A.; Yuan, Junjie; Pu, Hongting; Liu, Yongsheng (2010-03-24). "Newly UV-curable polyurethane coatings prepared by multifunctional thiol- and ene-terminated polyurethane aqueous dispersions: Photopolymerization properties". Polymer. 51 (7): 1572–1577. doi:10.1016/j.polymer.2010.02.003. ISSN 0032-3861.
- Yuan, Caideng; Wang, Mengyao; Li, Haitao; Wang, Zhongwei (2017-09-10). "Preparation and properties of UV-curable waterborne polyurethane-acrylate emulsion: ARTICLE". Journal of Applied Polymer Science. 134 (34): 45208. doi:10.1002/app.45208.
- Song, Sam Cha; Kim, Suk Joon; Park, Kyung-Kyu; Oh, Joong-Geul; Bae, Seong-Guk; Noh, Geon Ho; Lee, Won-Ki (2017-12-12). "Synthesis and properties of waterborne UV-curable polyurethane acrylates using functional isocyanate". Molecular Crystals and Liquid Crystals. 659 (1): 40–45. doi:10.1080/15421406.2018.1450824. ISSN 1542-1406. S2CID 102697178.
- Xu, Heping; Qiu, Fengxian; Wang, Yingying; Wu, Wenling; Yang, Dongya; Guo, Qing (2012-01-01). "UV-curable waterborne polyurethane-acrylate: preparation, characterization and properties". Progress in Organic Coatings. 73 (1): 47–53. doi:10.1016/j.porgcoat.2011.08.019. ISSN 0300-9440.
- Llorente, O.; Fernández-Berridi, M. J.; González, A.; Irusta, L. (2016-10-01). "Study of the crosslinking process of waterborne UV curable polyurethane acrylates". Progress in Organic Coatings. 99: 437–442. doi:10.1016/j.porgcoat.2016.06.020. ISSN 0300-9440.
- Dall Agnol, Lucas; Dias, Fernanda Trindade Gonzalez; Ornaghi, Heitor Luiz; Sangermano, Marco; Bianchi, Otávio (2021-05-01). "UV-curable waterborne polyurethane coatings: A state-of-the-art and recent advances review". Progress in Organic Coatings. 154: 106156. doi:10.1016/j.porgcoat.2021.106156. ISSN 0300-9440. S2CID 233544254.
- Li, Xiu; Wang, Di; Zhao, Longying; Hou, Xingzhou; Liu, Li; Feng, Bin; Li, Mengxin; Zheng, Pai; Zhao, Xuan; Wei, Shuangying (2021-02-01). "UV LED curable epoxy soybean-oil-based waterborne PUA resin for wood coatings". Progress in Organic Coatings. 151: 105942. doi:10.1016/j.porgcoat.2020.105942. ISSN 0300-9440. S2CID 225111943.
- Ahmed, Aziz; Sarkar, Preetom; Ahmad, Imtiaz; Das, Neeladri; Bhowmick, Anil K. (2015-01-14). "Influence of the Nature of Acrylates on the Reactivity, Structure, and Properties of Polyurethane Acrylates". Industrial & Engineering Chemistry Research. 54 (1): 47–54. doi:10.1021/ie502953u. ISSN 0888-5885.
- Li, Chunhong; Xiao, Hang; Wang, Xianfeng; Zhao, Tao (2018-04-10). "Development of green waterborne UV-curable vegetable oil-based urethane acrylate pigment prints adhesive: Preparation and application". Journal of Cleaner Production. 180: 272–279. doi:10.1016/j.jclepro.2018.01.193. ISSN 0959-6526.
- Li, Kaibin; Shen, Yiding; Fei, Guiqiang; Wang, Haihua; Li, Jingyi (2015-01-01). "Preparation and properties of castor oil/pentaerythritol triacrylate-based UV curable waterborne polyurethane acrylate". Progress in Organic Coatings. 78: 146–154. doi:10.1016/j.porgcoat.2014.09.012. ISSN 0300-9440.
- Xu, Jicheng; Jiang, Yan; Zhang, Tao; Dai, Yuting; Yang, Dongya; Qiu, Fengxian; Yu, Zongping; Yang, Pengfei (2018-05-01). "Fabrication of UV-curable waterborne fluorinated polyurethane-acrylate and its application for simulated iron cultural relic protection". Journal of Coatings Technology and Research. 15 (3): 535–541. doi:10.1007/s11998-017-0009-4. ISSN 1935-3804. S2CID 102688999.
- Javaheriannaghash, Hamid; Ghazavi, Nasrin (2012-05-01). "Preparation and characterization of water-based polyurethane–acrylic hybrid nanocomposite emulsion based on a new silane-containing acrylic macromonomer". Journal of Coatings Technology and Research. 9 (3): 323–336. doi:10.1007/s11998-011-9373-7. ISSN 1935-3804. S2CID 97951035. Archived from the original on 2023-03-14. Retrieved 2023-03-09.
- Phillips, Sidney L.; Troy Davis, M.; Phillips, Daniel J. (2004-10-01). "Mass transport mechanism for the formation of latex-modified epoxy coatings by evaporation from aqueous dispersions". JCT Research. 1 (4): 315–327. doi:10.1007/s11998-004-0033-z. ISSN 1935-3804. S2CID 98657583.
- Pradhan, Sukanya; Mohanty, Smita; Nayak, Sanjay K. (2018-05-01). "Effect of acrylation on the properties of waterborne epoxy: evaluation of physicochemical, thermal, mechanical and morphological properties". Journal of Coatings Technology and Research. 15 (3): 515–526. doi:10.1007/s11998-017-0006-7. ISSN 1935-3804. S2CID 139477266.
- Büyükyonga, Özge Naz; Akgün, Nagihan; Acar, Işıl; Güçlü, Gamze (2017-01-01). "Synthesis of four-component acrylic-modified water-reducible alkyd resin: investigation of dilution ratio effect on film properties and thermal behaviors". Journal of Coatings Technology and Research. 14 (1): 117–128. doi:10.1007/s11998-016-9835-z. ISSN 1935-3804. S2CID 99743427.
- Yu, Jianfeng; Pan, Hongxia; Zhou, Xiaodong (2014-05-01). "Preparation of waterborne phosphated acrylate–epoxy hybrid dispersions and their application as coil coating primer". Journal of Coatings Technology and Research. 11 (3): 361–369. doi:10.1007/s11998-013-9556-5. ISSN 1935-3804. S2CID 95541611. Archived from the original on 2023-03-14. Retrieved 2023-03-08.
- Zhu, Ke; Li, Xiaorui; Li, Jingyi; Wang, Haihua; Fei, Guiqiang (2017-11-01). "Properties and anticorrosion application of acrylic ester/epoxy core–shell emulsions: effects of epoxy value and crosslinking monomer". Journal of Coatings Technology and Research. 14 (6): 1315–1324. doi:10.1007/s11998-017-9930-9. ISSN 1935-3804. S2CID 103886023. Archived from the original on 2023-03-14. Retrieved 2023-02-20.
- Nichols, Mark (2014-03-01). "Waterborne coatings: continuing innovations". Journal of Coatings Technology and Research. 11 (2): 109. doi:10.1007/s11998-014-9574-y. ISSN 1935-3804. S2CID 136553869.
- Xie, Andong; Chen, Hongxiang; Xu, Caixia; Chen, Hailun; Zhou, Yu; Lin, Yinli; Yang, Minghua (2015-03-01). "Synthesis and characterization of waterborne polyurethane thickeners based on hyperbranched polyester". Journal of Coatings Technology and Research. 12 (2): 325–332. doi:10.1007/s11998-014-9636-1. ISSN 1935-3804. S2CID 95395939. Archived from the original on 2023-03-14. Retrieved 2023-03-08.
- Patil, Deepak M.; Phalak, Ganesh A.; Mhaske, S. T. (2017-03-01). "Synthesis of bio-based epoxy resin from gallic acid with various epoxy equivalent weights and its effects on coating properties". Journal of Coatings Technology and Research. 14 (2): 355–365. doi:10.1007/s11998-016-9853-x. ISSN 1935-3804. S2CID 100338583.
- Dai, Jinyue; Ma, Songqi; Wu, Yonggang; Zhu, Jin; Liu, Xiaoqing (2015-10-01). "High bio-based content waterborne UV-curable coatings with excellent adhesion and flexibility". Progress in Organic Coatings. 87: 197–203. doi:10.1016/j.porgcoat.2015.05.030. ISSN 0300-9440.
- Pradhan, Sukanya; Pandey, Priyanka; Mohanty, Smita; Nayak, Sanjay K. (2017-07-01). "Synthesis and characterization of waterborne epoxy derived from epoxidized soybean oil and bioderived C-36 dicarboxylic acid". Journal of Coatings Technology and Research. 14 (4): 915–926. doi:10.1007/s11998-016-9884-3. ISSN 1935-3804. S2CID 99038923.
- Franks, Felix (2012). The Physics and Physical Chemistry of Water. Springer. ISBN 978-1-4684-8334-5.
- Lide, David R. (2004). CRC Handbook of Chemistry and Physics (85th ed.). CRC Press. ISBN 978-0-8493-0485-9.
- Blank, Werner. "Formulating Polyurethane Dispersions" (PDF). Archived (PDF) from the original on 2017-08-28. Retrieved 2020-03-19.
- Asif, Anila; Huang, Chengyu; Shi, Wenfang (2003). "UV curing behaviors and hydrophilic characteristics of UV curable waterborne hyperbranched aliphatic polyesters". Polymers for Advanced Technologies. 14 (9): 609–615. doi:10.1002/pat.380. ISSN 1099-1581.
- "Floor Coatings with PUD" (PDF). Archived (PDF) from the original on 2018-11-01. Retrieved 2020-03-19.
- Howarth, G A; Manock, H L (July 1997). "Water-borne polyurethane dispersions and their use in functional coatings". Surface Coatings International. 80 (7): 324–328. doi:10.1007/bf02692680. ISSN 1356-0751. S2CID 137433262.
- "Waterborne Floor Coatings for Wood Floors" (PDF). Archived (PDF) from the original on 2018-11-01. Retrieved 2020-03-19.
- "PUD - Polymers - Adhesive Raw Materials - Adhesives - Markets & Industries - BASF Dispersions & Pigments". www.dispersions-pigments.basf.com. Archived from the original on 2020-03-19. Retrieved 2019-04-11.
- Howarth, GA (2003). "Polyurethanes, polyurethane dispersions and polyureas: Past, present and future". Surface Coatings International Part B: Coatings Transactions. 86 (2): 111–118. doi:10.1007/BF02699621. S2CID 93574741.
- US 5071904, Martin, Roxalana L.; Piccirilli, Barbara G. & Faler, Dennis L., "Waterborne coating compositions for automotive applications", published 1991-12-10, assigned to PPG Industries Inc.
- Communications, Covestro AG. "Automotive OEM Metal Metal Basecoat". www.coatings.covestro.com. Archived from the original on 2019-04-22. Retrieved 2019-04-22.
- "URESEAL - Water-Based High-Gloss Polyurethane Coating | Polygem Epoxy". www.polygem.com. Archived from the original on 2019-04-26. Retrieved 2019-04-26.
- Howarth G.A. “Waterborne epoxy resin systems for use in anti-corrosive primers” Pigment and Resin Technology Vol. 24 No 6 Nov./Dec. 1995 pp 3-6
- Howarth, G.A. and Hayward, G.R., “Waterborne Resins,” OCCA Student Monograph No. 3, Oil and Colour Chemists’ Association, UK, 1996.
- Christopher, Gnanaprakasam; Anbu Kulandainathan, Manickam; Harichandran, Gurusamy (2015-07-01). "Highly dispersive waterborne polyurethane/ZnO nanocomposites for corrosion protection". Journal of Coatings Technology and Research. 12 (4): 657–667. doi:10.1007/s11998-015-9674-3. ISSN 1935-3804. S2CID 136984192.
- Davim, J. Paulo (2012-10-16). The Design and Manufacture of Medical Devices. Cambridge, UK: Woodhead Publishing. p. 135. ISBN 9781907568725.
- Polymer modified cements and repair mortars. Daniels LJ, PhD thesis Lancaster University 1992
- Sikdar, Partha; Islam, Shafiqul; Dhar, Avik; Bhat, Gajanan; Hinchliffe, Doug; Condon, Brian (2022-07-01). "Barrier and mechanical properties of water-based polyurethane-coated hydroentangled cotton nonwovens". Journal of Coatings Technology and Research. 19 (4): 1255–1267. doi:10.1007/s11998-021-00609-3. ISSN 1935-3804. S2CID 247942460.
Further reading
- Epoxy resin technology. Paul F. Bruins, Polytechnic Institute of Brooklyn. New York: Interscience Publishers. 1968. ISBN 0-470-11390-1. OCLC 182890.
{{cite book}}: CS1 maint: others (link) - Flick, Ernest W. (1993). Epoxy resins, curing agents, compounds, and modifiers : an industrial guide. Park Ridge, NJ. ISBN 978-0-8155-1708-5. OCLC 915134542.
{{cite book}}: CS1 maint: location missing publisher (link) - Philip Sherman; British Society of Rheology (1963). Rheology of emulsions: proceedings of a symposium held by the British Society of Rheology ... Harrogate, October 1962. Macmillan. ISBN 9780080102900.
- Erbil, Yildirim H. (2000). Vinyl Acetate Emulsion Polymerization and Copolymerization with Acrylic Monomers. doi:10.1201/9781420038804. ISBN 9780429117794.
- Eliseeva, V. I. (1981). Emulsion Polymerization and Its Applications in Industry. S. S. Ivanchev, S. I. Kuchanov, A. V. Lebedev. Boston, MA: Springer US. ISBN 978-1-4684-1641-1. OCLC 851754165.
- Chern, Chorng-Shyan (2008). Principles and applications of emulsion polymerization. Hoboken, N.J.: Wiley. ISBN 978-0-470-37794-9. OCLC 264621081.
- Gooch, Jan W. (2002). Emulsification and polymerization of alkyd resins. New York: Kluwer Academic/Plenum Publishers. ISBN 0-306-47554-5. OCLC 51893677.
- Adel M. A. Mohamed (2016). Electrodeposition of Composite Materials. Teresa D. Golden. [Erscheinungsort nicht ermittelbar]. ISBN 978-953-51-4633-9. OCLC 1193046213.
{{cite book}}: CS1 maint: location missing publisher (link) - "Electrodeposition of Coatings"; American Chemical Society; Washington D.C.; 1973; ISBN 0-8412-0161-7
- Zhitomirsky, I. (2002-03-29). "Cathodic electrodeposition of ceramic and organoceramic materials. Fundamental aspects". Advances in Colloid and Interface Science. 97 (1): 279–317. doi:10.1016/S0001-8686(01)00068-9. ISSN 0001-8686. PMID 12027023.

.jpg.webp)