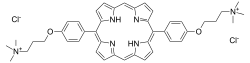
XF-73
 | |
| Identifiers | |
|---|---|
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C44H50Cl2N6O2 | |
| Molar mass | 765.82 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
XF-73 (Exeporfinium chloride) is an experimental drug candidate. It is an anti-microbial that works via weakening bacteria cell walls.[1] It is a potential treatment for methicillin-resistant Staphylococcus aureus (MRSA) and possibly Clostridium difficile. It is being developed by Destiny Pharma Ltd.[2][3][4]
Structurally, it is a dicationic porphyrin.[5]
It has completed a phase I clinical trial for nasal decolonisation of MRSA—being tested against 5 bacterial strains. It seems unlikely to cause MRSA to develop resistance to it.[1][6]
In 2014, a phase 1 clinical trial for nasal administration was run.[7]
As of June 2022, another phase 1 clinical trial (for nasal administration) completed recruiting in 2016 but no results have been posted.[8]
References
- Neka Sehgal (20 September 2010). "Promising new drug XF-73 kills superbugs within 5 minutes". Archived from the original on 21 July 2011.
- "XF-73". Destiny Pharma. 29 July 2016. (Shows molecular structure.)
- Miller, K.; Ooi, N.; Hobbs, J. K.; Rhys-Williams, W.; Love, W. G.; Hayter, I.; Katila, M.; Chopra, I. (19 April 2008). "XF-73, a novel anti-staphylococcal antimicrobial with very rapid bactericidal activity". European Society of Clinical Microbiology and Infectious Diseases.
{{cite journal}}: Cite journal requires|journal=(help) - Mary Dejevsky (18 May 2008). "Scientists 'on brink of cure' for superbug". The Independent. Archived from the original on 7 May 2022.
- "XF series". Destiny Pharma. 29 July 2016.
- Tom Chivers (18 May 2008). "MRSA: UK scientists 'close to a treatment". The Daily Telegraph. Archived from the original on 21 April 2013.
- Study of the Nasal Decolonisation of Staphylococcus Aureus (SA) and the Safety and Tolerability of XF-73 Nasal Gel in Healthy Subjects
- Study of the Safety and Local Tolerability of Intranasal Gel Formulations of XF-73