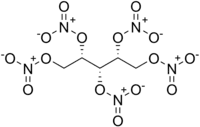
Xylitol pentanitrate
Xylitol pentanitrate (XPN) is a nitrated ester primary explosive[3][4] first synthesized in 1891 by Gabriel Bertrand.[5] Law enforcement has taken an interest in XPN along with erythritol tetranitrate (ETN) and pentaerythritol tetranitrate (PETN) due to their ease of synthesis, which makes them accessible to amateur chemists and terrorists.[6][7]
 | |
| Names | |
|---|---|
| IUPAC name
1,2,3,4,5-Pentakis-nitrooxy-pentane | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| |
| |
| Properties | |
| C5H7N5O15 | |
| Molar mass | 377.131 g·mol−1 |
| Density | 1.852 g/cm3 |
| Melting point | 45.5 °C (114 °F; 318 K) |
| Boiling point | 163-185 °C (346 - 358 °F; 436 - 458 K) (Decomposes) |
| Solubility | Soluble in ethanol, toluene, chloroform, acetone[1] |
| log P | 3.42[2] |
| Structure | |
| Monoclinic [3] | |
| Explosive data | |
| Shock sensitivity | 4.5 J[3] |
| Friction sensitivity | 18 N [3] |
| Detonation velocity | 7,100 m/s |
| Hazards | |
| GHS labelling: | |
  | |
| 167 °C (333 °F; 440 K) [3] | |
| Lethal dose or concentration (LD, LC): | |
LC50 (median concentration) |
148 (μM in splenocytes)[2] |
| Related compounds | |
Related compounds |
Xylitol Erythritol tetranitrate Pentaerythritol tetranitrate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Properties
At room temperature XPN exists as a white crystalline solid. When heated to 163 °C, liquid xylitol pentanitrate begins to crackle and produce a dark vapour. When decomposed, every gram of XPN produces 200 mL of gas, which makes it a high performance explosive.[3]
Rotter impact analysis of XPN found a figure of insensitiveness of 25 (RDX = 80). XPN displayed a similar sensitivity to electric static discharge to ETN and PETN.[3]
Synthesis
Xylitol pentanitrate is formed by reaction of xylitol pentaacetate with fuming nitric acid and glacial acetic acid.[5]
Complete oxidation
Much like ETN, XPN has a positive oxygen balance, which means the carbon and oxygen in the molecule can be fully oxidized without another oxidizing agent being added.
The decomposition of four molecules of XPN releases three O2. The free oxygen molecules can be used to oxidize an added metal dust or negative oxygen balanced explosive like TNT.
See also
- Nitroglycerine
- Xylitan trinitrate ([(2R,3S,4S)-3,4-dinitrooxyoxolan-2-yl]methyl nitrate) – used as plasticizer in propellants similarly to nitroglycerine
- Mannitol hexanitrate
References
- Stark, Kelly-Anne S.; Gascooke, Jason R.; Gibson, Christopher T.; Lenehan, Claire E.; Bonnar, Callum; Fitzgerald, Mark; Kirkbride, K. Paul (November 2020). "Xylitol pentanitrate – Its characterization and analysis". Forensic Science International. 316: 110472. doi:10.1016/j.forsciint.2020.110472. PMID 32919164. S2CID 221643415.
- Šarlauskas, Jonas; KrikŠtopaitis, Kastis; MiliukienĖ, Valė; ČĖnas, Narimantas; AnuseviČius, Žilvinas; ŠaikŪnas, Algirdas (2011). "Investigation on the Electrochemistry and Cytotoxicity of Organic Nitrates and Nitroamines". Central European Journal of Energetic Materials. 8: 15–24.
- Stark, Kelly‐Anne S.; Alvino, Jason F.; Kirkbride, K. Paul; Sumby, Christopher J.; Metha, Gregory F.; Lenehan, Claire E.; Fitzgerald, Mark; Wall, Craig; Mitchell, Mark; Prior, Chad (2019). "Crystal Structure, Sensitiveness and Theoretical Explosive Performance of Xylitol Pentanitrate (XPN)". Propellants, Explosives, Pyrotechnics. 44 (5): 541–549. doi:10.1002/prep.201800337. ISSN 0721-3115. S2CID 109872121.
- Klapötke, Thomas M. (2021-01-18). "X". O-Z. De Gruyter. pp. 2027–2030. doi:10.1515/9783110672558-019. ISBN 978-3-11-067255-8.
- Wright, I. G.; Hayward, L. D. (1960). "The Pentitol Pentanitrates". Canadian Journal of Chemistry. 38 (2): 316–319. doi:10.1139/v60-045. ISSN 0008-4042.
- Yan, Qi-Long; Künzel, Martin; Zeman, Svatopluk; Svoboda, Roman; Bartošková, Monika (2013). "The effect of molecular structure on thermal stability, decomposition kinetics and reaction models of nitric esters". Thermochimica Acta. 566: 137–148. doi:10.1016/j.tca.2013.05.032.
- Dong, Jun; Yan, Qi-Long; Liu, Pei-Jin; He, Wei; Qi, Xiao-Fei; Zeman, Svatopluk (2018). "The correlations among detonation velocity, heat of combustion, thermal stability and decomposition kinetics of nitric esters". Journal of Thermal Analysis and Calorimetry. 131 (2): 1391–1403. doi:10.1007/s10973-017-6706-5. ISSN 1388-6150. S2CID 102678177.
External links
 Media related to Xylitol pentanitrate at Wikimedia Commons
Media related to Xylitol pentanitrate at Wikimedia Commons