YOYO-1
YOYO-1 is a green fluorescent dye used in DNA staining.[1] It belongs to the family of monomethine cyanine dyes and is a tetracationic homodimer of Oxazole Yellow (abbreviated YO, hence the name YOYO), typically available as tetraiodide salt. In aqueous buffer, free YOYO-1 dye (absorption: λmax 458 nm, emission: λmax 564 nm) has very low fluorescence quantum yield. However, the intensity of fluorescence increases 3200 times upon binding through bis-intercalation to double-stranded DNA (absorption: λmax 489 nm, emission: λmax 509 nm).[2]
| Names | |
|---|---|
| Preferred IUPAC name
[12(2)Z,16(172)Z]-13,7,7,11,11,173-Hexamethyl-13H,173H-7,11-diaza-31λ5,151λ5-3(4,1),15(1,4)-diquinolina-1,17(2)-bis([1,3]benzoxazola)heptadecaphane-12(2),16(172)-diene-7,11-diium-31,151-bis(ylium) tetraiodide | |
| Other names
YOYO, YOYO-1, YoYo-1 | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C49H58I4N6O2 | |
| Molar mass | 1270.642 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Synthesis
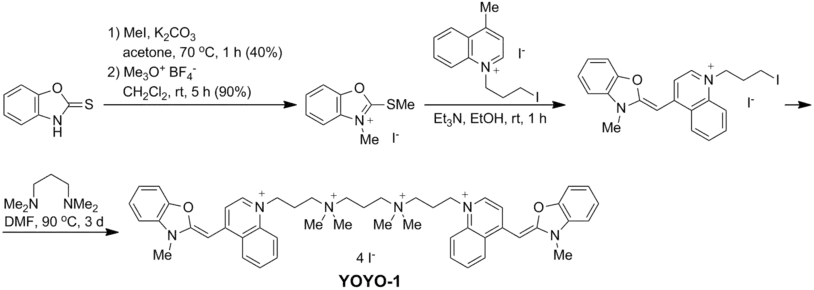
YOYO-1 is prepared by alkylation of N,N,N′,N′-tetramethyl-1,3-propanediamine with 2 equivalents of N-(3-iodopropyl) analogue of Oxazole Yellow,[2] which is available in three steps from 2-mercaptobenzoxazole:[3]
Photophysics
The molar attenuation coefficient at the absorption peak of YOYO-1 is nearly 105 cm−1M−1, among the high values of typical organic dyes. The fluorescence quantum yield of YOYO-1 in water is very small (<0.1%) and thus nonfluorescent. Upon binding to DNA, its quantum yield enhances >1000 fold and reaches up to 50%, among the brightest fluorescent organic dyes.
Under light excitation, photoblinking and photobleaching are observed for YOYO-1 in DNA. The latter is also believed to be the cause of the photocleavage of the host DNA molecules by generating reactive free radicals.
Two major mechanisms have been proposed in the literature to explain why YOYO-1 is not fluorescent in the polar solvents but is fluorescent when intercalated in the DNA base pairs. The first one is intramolecular charge transfer and the second is intermolecular charge transfer. Both are still actively under examination on which one being dominating.
The intramolecular charge transfer mechanism was established around the 1980s. Under light illumination in water, the excited electron in the molecule shifts its probability across the methine group which enables the molecule to rotate along with the methine group, a photoisomerization reaction. This rotation relaxes the energy nonradiatively thus the molecule is not fluorescent. However, when YOYO-1 is intercalated into the DNA base pairs, it gets stuck and cannot rotate. Thus the molecule stays fluorescent. The major evidence is when increase the viscosity of the solution by cooling down and increase the glucose content in a mixed solution, the quantum yield of YOYO-1 increases.
The intermolecular charge transfer mechanism was proposed in 2018.[4] YOYO-1 is weakly bonded with the polar solvent molecules. When it is excited by light, an electron-hole pair is created in the molecule. The hole is able to get an extra electron from the solvent that created a negatively charged YOYO-1 radical and a positively charged radical in the solution. Most time the radical neutralize each other very quickly and nonradiatively. This is a Dexter electron transfer process that quenches the fluorescence and creates the rotation of the YOYO-1 molecule. As such, the rotation is a product of the quenching, not the cause of the quenching proposed in the intramolecular charge transfer mechanism. There is a small probability that the radicals separate each other and diffuse away, which then makes the YOYO-1 molecule susceptible to photobleaching. When the YOYO-1 molecules are intercalated in the DNA molecule, the hydrophobic base pairs greatly reduce the radical formation because DNA is a poor electrical conductor. Thus the fluorescent quenching by the solvent is greatly reduced. The small number of leaking charges create long-lived radicals and cut the DNA backbones that results in a DNA photocleavage. This mechanism has been supported by the data in femtosecond ultrafast transient absorption spectroscopy.
If the intramolecular charge transfer mechanism dominates the fluorescent quenching of YOYO-1 in water, then one can add bulky ligand to stop or slow down the rotation to increase its quantum yield in water. However, if the intermolecular charge transfer dominates, one can modify the molecule to stop the charge transfer between YOYO-1 and water either by charge passivation, or ligand modification to shift its redox potentials.
Applications
The major applications of YOYO-1 are for DNA staining. Under normal conditions and pH level, the four positive charge of each YOYO-1 molecule makes it very easy to bind to DNA molecules which have negatively charged backbones. The dye then readily intercalates into the DNA either with one end or two ends depending on the annealing temperature and room available. The DNA then lights up under a fluorescence microscope with very small background from the unbound YOYO-1. The measurement can be at bulk ensemble level or at the single-molecule level. The latter has enabled ultra-high resolution using a super-resolution microscopy.[5] With high illumination power, the dye is known to generate free radicals which then photocleave DNA strands.
References
- Bennink, ML; Schärer, OD; Kanaar, R; Sakata-Sogawa, K; et al. (June 1999). "Single-molecule manipulation of double-stranded DNA using optical tweezers: Interaction studies of DNA with RecA and YOYO-1". Cytometry Part A. 36 (3): 200–208. doi:10.1002/(SICI)1097-0320(19990701)36:3<200::AID-CYTO9>3.0.CO;2-T. PMID 10404969.
- Rye, HS; Yue, S; Wemmer, DE; Quesada, MA; et al. (1992). "Stable fluorescent complexes of double-stranded DNA with bis-intercalating asymmetric cyanine dyes: properties and applications". Nucleic Acids Research. 20 (11): 2803–2812. doi:10.1093/nar/20.11.2803. PMC 336925. PMID 1614866.
- WO 2010141833, Lee Josephson; Elisabeth Garanger & Scott Hilderbrand et al., "Vital fluorochrome conjugates and methods of use", published 2010-12-09, assigned to The General Hospital Corp
- Wang, L; Pyle, JR; Cimatu, KA; Chen, J (1 December 2018). "Ultrafast Transient Absorption Spectra of Photoexcited YOYO-1 molecules call for additional investigations of their fluorescence quenching mechanism". Journal of Photochemistry and Photobiology A: Chemistry. 367: 411–419. doi:10.1016/j.jphotochem.2018.09.012. PMC 6217845. PMID 30410276.
- Pyle, Joseph R; Chen, Jixin (2 November 2017). "Photobleaching of YOYO-1 in super-resolution single DNA fluorescence imaging". Beilstein Journal of Nanotechnology. 8: 2296–2306. doi:10.3762/bjnano.8.229. PMC 5687005. PMID 29181286.