Zerovalent iron
Zerovalent iron (ZVI) is jargon that describes forms of iron metal that are proposed for used in Groundwater remediation.[1][2][3][4]

Model A
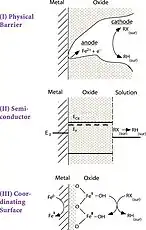
Model B
Click either image to enlarge
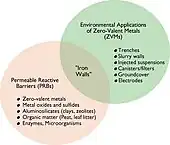
Venn diagram showing the overlap between ZVIs and PRBs
ZVI operates by electron transfer from Fe0 toward some organochlorine compounds, a common class of pollutants. The remediation process is proposed to generate Fe2+ and Cl− and halide-free organic products, all of which are relatively innocuous.[5] The technology is not however been implemented, despite many proofs of principle.
Type of ZVI
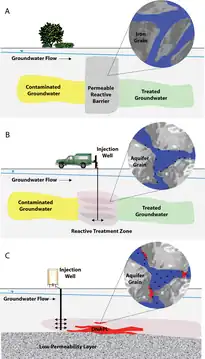
click to enlarge
- Bulk Fe. Cast iron, consisting of scrap iron of construction grade, has been used as a reactive material for permeable reactive barriers for groundwater remediation. Reactions are generally believed to occur on the Fe (oxide) surface; however, graphite inclusions have been shown can also serve as a reaction sites.[6]
- Nanoscale Fe. In addition to using macroscale iron in PRBs, nanoparticles (1-100 nm diameter) of zerovalent iron (nZVI) are effective.[2]
- Zn. Zinc has showed much higher reactivity toward pentachlorophenol than iron. This indicates that zinc may be used as a replacement for ZVI in dechlorinating chlorinated phenols. Chlorinated phenols are sequentially dechlorinated and thus less chlorinated phenols have been identified as a reduction product.[7]
Type of contaminants treated
Many kinds of pollutants have been proposed, but few have been demonstrated in solving environmental challenges.
- Cadmium (Cd2+) is converted to immobile Cd metal.[8]
- Chloramines are effectively reduced by ZVI.[9]
- nitrate reduction by iron powder is observed only at pH≤4.[10] Ammonia is the end product. Using nanoscale iron N2 gas is the product.[11]
- Nitrated aromatics are reduced by bulk iron.[6][12][13]
- Chlorinated pesticides such as DDT, DDD, and DDE. The rates of dechlorination are enhanced by the surfactant (Triton X-114).[14]
Further reading
- Tratnyek, P. G.; M. M. Scherer; T. J. Johnson; Matheson, L.J. (2003). Permeable reactive barriers of iron and other zerovalent metals. In: Tarr M. A. (ed.), Chemical Degradation Methods for Wastes and Pollutants; Environmental and Industrial Applications. Environmental Science and Pollution Control, Marcel Dekker, New York, pp 371–421. doi:10.1201/9780203912553.ch9
Notes
- Fu, Fenglian; Dionysiou, Dionysios D.; Liu, Hong (2014). "The use of zero-valent iron for groundwater remediation and wastewater treatment: A review". Journal of Hazardous Materials. 267: 194–205. doi:10.1016/j.jhazmat.2013.12.062.
- Li, Xiao-qin; Elliott, Daniel W.; Zhang, Wei-Xian (2006). "Zero-Valent Iron Nanoparticles for Abatement of Environmental Pollutants: Materials and Engineering Aspects". Critical Reviews in Solid State and Materials Sciences. 31 (4): 111–122. Bibcode:2006CRSSM..31..111L. doi:10.1080/10408430601057611. S2CID 4834565.
- Stefaniuk, Magdalena; Oleszczuk, Patryk; Ok, Yong Sik (2016). "Review on nano zerovalent iron (NZVI): From synthesis to environmental applications". Chemical Engineering Journal. 287: 618–632. doi:10.1016/j.cej.2015.11.046.
- Gillham, Robert, John Vogan, Lai Gui, Michael Duchene, and Jennifer Son. "Iron Barrier Walls for Chlorinated Solvent Remediation." In Situ Remediation of Chlorinated Solvent Plumes. Ed. Hans F. Stroo and C. Herb Ward. New York, NY: Springer Science+Business Media, 2010.
- Tratnyek, Paul, and Rick Johnson. "Remediation with Iron Metal." Center for Groundwater Research. Oregon Health and Science University, 04 Feb. 2005.
- Jafarpour, B.; Imhoff, P. T.; Chiu. P.C. 2005. Quantification and modeling of 2,4-dinitrotoluene reduction with high-purity and cast iron. Journal of Contaminant Hydrology. 76(1-2): 87-107. doi:10.1016/j.jconhyd.2004.08.001
- Kim, Y. H.; Carraway, E. R. 2003. Dechlorination of chlorinated phenols by zerovalent zinc. Environmental Technology. 24(12): 1455-1463. doi:10.1080/09593330309385690
- Boparai, Hardiljeet K.; Joseph, Meera; o'Carroll, Denis M. (2011). "Kinetics and thermodynamics of cadmium ion removal by adsorption onto nano zerovalent iron particles". Journal of Hazardous Materials. 186 (1): 458–465. doi:10.1016/j.jhazmat.2010.11.029. PMID 21130566.
- Bedner, M.; W. A. MacCrehan; G. R. Helz. 2004. Making chlorine greener: investigation of alternatives to sulfite for dechlorination. Water Research. 38(10): 2505-2514. doi:10.1016/j.watres.2004.03.010
- Huang, C.; Wang, H.; Chiu, P. 1998. Nitrate reduction by metallic iron. Water Research. 32(8): 2257-2264. doi:10.1016/S0043-1354(97)00464-8
- Choe, S.; Chang, Y.; Hwang, K.; Khim, J. 1999. Kinetics of reductive denitrification by nanoscale zerovalent iron. Chemosphere. 41(8): 1307-1311. doi:10.1016/S0045-6535(99)00506-8
- Mahood, S. A.; Schaffner\doi=10.15227/orgsyn.011.0032, P. V. L. (1931). "2,4-Diaminotoluene". Organic Syntheses. 11: 32.
- "O-Aminobenzaldehyde, Redox-Neutral Aminal Formation and Synthesis of Deoxyvasicinone". Organic Syntheses. 89: 274. 2012. doi:10.15227/orgsyn.089.0274.
- Sayles, G. D.; You, G.; Wang, M.; Kupferle, M. J. 1997. DDT, DDD, and DDE dechlorination by zerovalent iron. Environmental Science & Technology. 31(12): 3448-3454. doi:10.1021/es9701669
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.