Acetyl chloride
Acetyl chloride (CH3COCl) is an acyl chloride derived from acetic acid. It belongs to the class of organic compounds called acid halides. It is a colorless, corrosive, volatile liquid. Its formula is commonly abbreviated to AcCl.
| |||
 | |||
 | |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Acetyl chloride[2] | |||
| Systematic IUPAC name
Ethanoyl chloride | |||
| Other names
Acyl chloride | |||
| Identifiers | |||
CAS Number |
|||
3D model (JSmol) |
|||
Beilstein Reference |
605303 | ||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.000.787 | ||
| EC Number |
| ||
Gmelin Reference |
1611 | ||
PubChem CID |
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 1717 | ||
CompTox Dashboard (EPA) |
|||
InChI
| |||
SMILES
| |||
| Properties | |||
Chemical formula |
CH3COCl | ||
| Molar mass | 78.49 g/mol | ||
| Appearance | Colorless liquid | ||
| Density | 1.104 g/ml, liquid | ||
| Melting point | −112 °C (−170 °F; 161 K) | ||
| Boiling point | 52 °C (126 °F; 325 K) | ||
Solubility in water |
Reacts with water | ||
Magnetic susceptibility (χ) |
-38.9·10−6 cm3/mol | ||
| Structure | |||
Dipole moment |
2.45 D | ||
| Hazards | |||
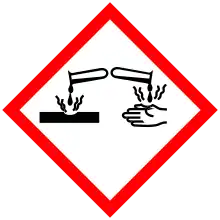
| GHS labelling: | |||
Pictograms |
   | ||
Signal word |
Danger | ||
Hazard statements |
H225, H302, H314, H335, H412 | ||
Precautionary statements |
P210, P233, P240, P241, P242, P243, P260, P261, P264, P270, P271, P273, P280, P301+P312, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P310, P312, P321, P330, P363, P370+P378, P403+P233, P403+P235, P405, P501 | ||
| NFPA 704 (fire diamond) | |||
| Flash point | 4 °C (39 °F; 277 K) | ||
Autoignition temperature |
390 °C (734 °F; 663 K) | ||
| Explosive limits | 7.3–19% | ||
| Related compounds | |||
Related acyl chlorides |
Propionyl chloride Butyryl chloride | ||
Related compounds |
Acetic acid Acetic anhydride Acetyl bromide | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |||
Synthesis
On an industrial scale, the reaction of acetic anhydride with hydrogen chloride produces a mixture of acetyl chloride and acetic acid:[3]
- (CH3CO)2O + HCl → CH3COCl + CH3CO2H
Laboratory routes
Acetyl chloride was first prepared in 1852 by French chemist Charles Gerhardt by treating potassium acetate with phosphoryl chloride.[4]
Acetyl chloride is produced in the laboratory by the reaction of acetic acid with chlorodehydrating agents such as PCl3, PCl5, SO2Cl2, phosgene, or SOCl2. However, these methods usually give acetyl chloride contaminated by phosphorus or sulfur impurities, which may interfere with the organic reactions.[5]
Other methods
When heated, a mixture of dichloroacetyl chloride and acetic acid gives acetyl chloride.[5] It can also be synthesized from the catalytic carbonylation of methyl chloride.[6] It also arises from the reaction of acetic acid, acetonitrile, and hydrogen chloride.
Occurrence
Acetyl chloride is not expected to exist in nature, because contact with water would hydrolyze it into acetic acid and hydrogen chloride. In fact, if handled in open air it releases white "smoke" resulting from hydrolysis due to the moisture in the air. The smoke is actually small droplets of hydrochloric acid and acetic acid formed by hydrolysis.
Uses
Acetyl chloride is used for acetylation reactions, i.e., the introduction of an acetyl group. Acetyl is an acyl group having the formula −C(=O)−CH3). For further information on the types of chemical reactions compounds such as acetyl chloride can undergo, see acyl halide. Two major classes of acetylations include esterification and the Friedel-Crafts reaction.
Acetic acid esters and amide
Acetyl chloride is a reagent for the preparation of esters and amides of acetic acid, used in the derivatization of alcohols and amines. One class of acetylation reactions are esterification, for example the reaction with ethanol to produce ethyl acetate and hydrogen chloride:
Frequently such acylations are carried out in the presence of a base such as pyridine, triethylamine, or DMAP, which act as catalysts to help promote the reaction and as bases neutralize the resulting HCl. Such reactions will often proceed via ketene.
Friedel-Crafts acetylations
A second major class of acetylation reactions are the Friedel-Crafts reactions.[7]
See also
- Acetic acid
- Acetyl bromide
- Acetyl fluoride
- Acetyl iodide
References
- Merck Index, 11th Edition, 79.
- Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. pp. 796–797. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- Cheung, Hosea; Tanke, Robin S.; Torrence, G. Paul (2000). "Acetic Acid". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a01_045.
- See:
- Gerhardt, Charles (1852) "Ueber wasserfreie organische Säuren" (On anhydrous organic acids), Annalen der Chemie und Pharmacie, 83 : 112–116.
- Gerhardt, Charles (1853) "Untersuchungen über die wasserfreien organischen Säuren" (Investigations into anhydrous organic acids), Annalen der Chemie und Pharmacie, 87 : 57–84 ; see especially pp. 68–71.
- Leo A. Paquette (2005). "Acetyl chloride". Handbook of Reagents for Organic Synthesis, Activating Agents and Protective Groups. John Wiley & Sons. p. 16. ISBN 978-0-471-97927-2.
- US 4352761, Erpenbach, Heinz; Gehrmann, Klaus & Lork, Winfried et al., "Production of acetyl chloride", published 1982-10-05, assigned to Hoechst AG
- Charles Merritt, Jr and Charles E. Braun "9-Acetylanthracene" Org. Synth. 1950, 30, 2. doi:10.15227/orgsyn.030.0001