Adegramotide
Adegramotide (DSP-7888) is an experimental drug intended for treatment of glioblastoma multiforme.[1] It is a peptide vaccine and, as of 2017, in phase II clinical trials.[2][3]
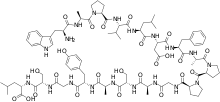 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
| Formula | C87H123N19O24 |
| Molar mass | 1819.050 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
References
- Spira A, Hansen AR, Harb WA, Curtis KK, Koga-Yamakawa E, Origuchi M, Li Z, Ertik B, Shaib WL (July 2021). "Multicenter, Open-Label, Phase I Study of DSP-7888 Dosing Emulsion in Patients with Advanced Malignancies". Targeted Oncology. 16 (4): 461–469. doi:10.1007/s11523-021-00813-6. PMC 8266707. PMID 33939067.
- Boston Biomedical (11 May 2017). "A Study of DSP-7888 Dosing Emulsion in Combination With Bevacizumab in Patients With Recurrent or Progressive Glioblastoma Following Initial Therapy". ClinicalTrials.gov. U.S. National Library of Medicine.
- "Adegrapepimut-S". pubchem.ncbi.nlm.nih.gov.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.