Aromatic L-amino acid decarboxylase deficiency
Aromatic L-amino acid decarboxylase deficiency is a rare genetic disorder caused by mutations in the DDC gene, which encodes an enzyme called aromatic L-amino acid decarboxylase.
| Aromatic L-amino acid decarboxylase deficiency | |
|---|---|
| Specialty | Neurology |
| Symptoms | Dystonia, oculogyric crises, developmental delay, parkinsonism, dysautonomia |
| Usual onset | Infancy |
| Causes | Autosomal recessive DDC mutation |
| Diagnostic method | Lumbar puncture for neurotransmitter analysis; enzyme assay; genetic testing |
Signs and symptoms

Babies with severe aromatic L-amino acid decarboxylase deficiency usually present during the first few months of life. Symptoms can include:
- Hypotonia (floppiness)
- Developmental delay
- Oculogyric crises[2]
- Difficulty with initiating and controlling movements
- Dystonia and dyskinesia
- Gastointestinal dysmotility which can present at as vomiting, gastro-oesophageal reflux, diarrhoea and/or constipation
- Autonomic symptoms including difficulties controlling temperature and blood sugar, excessive sweating and nasal congestion
Some people may develop cerebral folate deficiency, because O-methylation of the excessive amounts of L-Dopa can deplete methyl donors such as S-adenosyl methionine and levomefolic acid. This deviation can be detected by measuring the levels of levomefolic acid in the cerebrospinal fluid, and can be corrected by folinic acid.[1]
Genetics
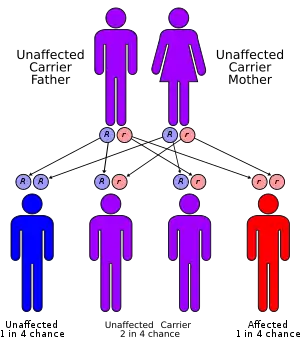
Aromatic L-amino acid decarboxylase deficiency is an autosomal recessive condition, meaning an individual needs to have two faulty copies of the DDC gene in order to be affected. Usually, one copy is inherited from each parent.[3]
Pathophysiology
The aromatic L-amino acid decarboxylase deficiency enzyme is involved in the synthesis of dopamine and serotonin, both of which are important neurotransmitters.
Diagnosis
Once there is a clinical suspicion of the diagnosis, neurotransmitters can be analysed in cerebrospinal fluid from a lumbar puncture. If these show the pattern of abnormalities typical for aromatic L-amino acid decarboxylase deficiency, the diagnosis can be confirmed by genetic testing and/or measurement of enzyme activity.
Treatment
There is no cure for aromatic L-amino acid decarboxylase deficiency, but medical and multidisciplinary treatment can relieve some of the symptoms. Individuals will require physiotherapy, occupational therapy, and speech and language therapy. Some will need enteral feeding (for example, a gastrostomy or jejunostomy) due to difficulties with chewing and swallowing.
Various medications can help compensate for the missing neurotransmitters. Dopamine agonists such as rotigotine or pramipexole and monoamine oxidase inhibitors such as selegiline are commonly used. Individuals may also need to take a range of other medications to control dyskinesia, constipation and other symptoms.[1]
In July 2021, results of a small gene therapy phase I study reported observation of dopamine restoration on seven participants between 4 and 9 years old.[4][5][6]
As of May 2022, the gene therapy product eladocagene exuparvovec is recommended for approval by the European Commission.[7][8]
References
- Wassenberg T, Molero-Luis M, Jeltsch K, Hoffmann GF, Assmann B, Blau N, et al. (January 2017). "Consensus guideline for the diagnosis and treatment of aromatic l-amino acid decarboxylase (AADC) deficiency". Orphanet J Rare Dis. 12 (1): 12. doi:10.1186/s13023-016-0522-z. PMC 5241937. PMID 28100251.
- Korenke, GC; Christen, HJ; Hyland, K; Hunneman, DH; Hanefeld, F (1997). "Aromatic L-amino acid decarboxylase deficiency: an extrapyramidal movement disorder with oculogyric crises". Eur J Paediatr Neurol. 1 (2–3): 67–71. doi:10.1016/S1090-3798(97)80065-7. PMID 10728198.
- Montioli, Riccardo; Dindo, Mirco; Giorgetti, Alejandro; Piccoli, Stefano; Cellini, Barbara; Voltattorni, Carla Borri (2014). "A comprehensive picture of the mutations associated with aromatic amino acid decarboxylase deficiency: From molecular mechanisms to therapy implications". Human Molecular Genetics. 23 (20): 5429–5440. doi:10.1093/hmg/ddu266. PMID 24865461.
- Pearson TS, Gupta N, San Sebastian W, Imamura-Ching J, Viehoever A, Grijalvo-Perez A, et al. (July 2021). "Gene therapy for aromatic L-amino acid decarboxylase deficiency by MR-guided direct delivery of AAV2-AADC to midbrain dopaminergic neurons". Nature Communications. 12 (1): 4251. Bibcode:2021NatCo..12.4251P. doi:10.1038/s41467-021-24524-8. ISSN 2041-1723. PMC 8275582. PMID 34253733.
- Ibrahim, Mennatalla (2021-07-14). "Gene therapy restores missing dopamine in children with rare brain disease". Science. Retrieved 2021-07-18.
- "Gene therapy trial points to a wider window to alter course of rare disease". STAT. 2021-07-12. Retrieved 2021-07-18.
- "Upstaza: Pending EC decision". European Medicines Agency. 19 May 2022. Retrieved 22 May 2022.
- "PTC Therapeutics Receives Positive CHMP Opinion for Upstaza for the Treatment of AADC Deficiency". PTC Therapeutics (Press release). 20 May 2022. Retrieved 22 May 2022.