Cafeteria roenbergensis
Cafeteria roenbergensis is a small bacterivorous marine flagellate. It was discovered by Danish marine ecologist Tom Fenchel and named by him and taxonomist David J. Patterson in 1988. It is in one of three genera of bicosoecids, and the first discovered of two known Cafeteria species. Bicosoecids belong to a broad group, the stramenopiles, also known as heterokonts (Heterokonta) that includes photosynthetic groups such as diatoms, brown, and golden algae, and non-photosynthetic groups such as opalinids, actinophryid "heliozoans", and oomycetes. The species is found primarily in coastal waters where there are high concentrations of bacteria on which it grazes. Its voracious appetite plays a significant role in regulating bacteria populations.[2]
| Cafeteria roenbergensis | |
|---|---|
 | |
| Scientific classification | |
| Kingdom: | Chromista |
| Phylum: | Bigyra |
| Class: | Bicosoecophyceae |
| Order: | Bicosoecales |
| Family: | Cafeteriaceae |
| Genus: | Cafeteria Fenchel & Patterson, 1988 |
| Species: | C. roenbergensis |
| Binomial name | |
| Cafeteria roenbergensis Fenchel & Patterson, 1988[1] | |
Physiology
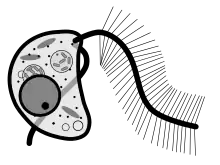
Cafeteria roenbergensis is a slightly flattened, kidney-shaped bicosoecid. Its cell typically measures between 3 and 10 μm and it has a volume of around 20 μm³.[3] It is colorless and has two unequally sized flagella. The smooth flagellum, angled posteriorly, is shorter, and attaches to substrates in non-motile cells, but trails behind in motile cells. The hairy flagellum points forward in an arc in sessile cells. Cafeteria is a eukaryotic organism, so it contains the typical organelles such as mitochondria and nuclei.[3]
Cafeteria roenbergensis reproduces asexually via binary fission, first replicating the flagella and internal organelles before the cell divides. No sexual activity is known for this species. Cells can replicate in under 10 hours.
Behavior
Cafeteria roenbergensis is a suspension feeder, meaning that it feeds by filtering suspended bacteria, its primary food source, and other particulate matter from the water.[4] Its two flagella facilitate feeding, locomotion and attachment to substrates. The anterior flagellum is responsible for locomotion and feeding. It propels the cell in a swift spiral movement. During feeding, it beats at about 40 times per second to create a current of water that moves about 100 micrometers/second. This current brings bacteria to its mouthparts. The food is ingested below the base of the flagella, which is referred to as the ventral side. In nonmotile C. roenbergensis cells, (cells that prefer to anchor themselves to a substrate) the posterior flagellum helps attach the organism to a substrate while it is feeding.[5] The flagella are anchored by 'rootlets' ribbons and subcellular ropes. They act as a skeleton and also support the mouth region.
Ecology
Bacterivorous nanoflagellates, the general group to which C. roenbergensis belongs, make up a significant portion of the oceans' protozoan communities, as well as those in freshwater, soils and other habitats. They are reported to be the primary consumer of bacteria in many habitats, controlling bacterial populations as they "graze".[6]
Habitat
Cafeteria roenbergensis has been found in all oceans examined, but is especially common in coastal waters.[2] These protists occur in a type of biosphere known as "microbial assemblages". This means that they are present at such low abundances, that they are not easily detected, and can only be retrieved and isolated using specialized isolation techniques such as flow cytometry.[7] Ishigaki and Sleigh (2001) found that C. roenbergensis ceased to reproduce when the concentration of bacteria that they were grazing on became less than 2.0×107 cells ml−1.[8] Other flagellates were able to multiply at much lower bacterial concentrations, indicating that bacterial concentration is a limiting factor for Cafeteria. Flagellates have varying abilities to gather bacteria to their mouths with their flagella, and this study suggests that the abilities of Cafeteria species may be inferior to other flagellates, since Cafeteria are usually specific to niches with high concentrations of bacteria.[4]
Virus
A giant virus, Cafeteria roenbergensis virus (CroV) infects and causes the lysis of C. roenbergensis.[9] The impact of CroV on natural populations of C. roenbergensis remains unknown; however, the virus has been found to be very host specific, and does not infect other closely related organisms.[4] Cafeteria roenbergensis is also infected by a second virus, the Mavirus virophage, which is only able to replicate in the presence of CroV.[10] This virus interferes with the replication of CroV, which leads to the survival of C. roenbergensis cells. Mavirus is able to integrate into the genome of cells of C. roenbergensis, and thereby confer immunity to the population [11]
Taxonomy
Cafeteria is categorized in a group called the "Heterotrophic group". It has one other known species in its genus, Cafeteria minuta, which was found living in tropical marine sediments by Larsen and Patterson in 1990.[6]
Name
Marine biologist Tom Fenchel, one of the two species authorities who first described C. roenbergensis, is credited with having joked about the chromalveolate's name: "We found a new species of ciliate during a marine field course in Rønbjerg and named it Cafeteria roenbergensis because of its voracious and indiscriminate appetite after many dinner discussions in the local cafeteria."[12]
Mitochondrial genome
Cafeteria roenbergensis has a highly compact mitochondrial genome that includes less than 3.4% introns. Some sources hold that its mitochondrial genome contains no introns at all.[13] The mitochondrial translation code in C. roenbergensis is not standard in comparison to its closest known relatives, Phytophthora infestans and Ochromonas danica. Instead of acting as a stop codon, in Cafeteria, UGA codes for tryptophan.[14]
Culture
Because they are easy to grow in culture, Cafeteria roenbergensis has been subject to a diversity of more detailed studies, such as genomic and ecological studies that have revealed that this species has the most functionally compact DNA amongst eukaryotes.[2] While in culture, Cafeteria are fed Vibrio bacteria. In a test conducted by Park and Simpson in 2010, it was found that Cafeteria cells grow best in salinities of 3 ppm to 100 ppm, but cannot survive at concentrations any higher.[15]
References
- Fenchel, T., Patterson, D. J. (1988). Cafeteria roenbergensis nov. gen., nov. sp., a heterotrophic microflagellate from marine plankton. Marine Microbial Food Webs, 3, 9–19, .
- O'Kelly, C. J.; G. Burger (29 July 1994). "Cafeteria roenbergensis MtDNA". Evolutionary & Integrative Genomics. PID. Retrieved 8 January 2012.
- Otto, K.; D. Weichart & S. Kjelleberg (1997). "Plasmid transfer between marine Vibrio strains during predation by the heterotrophic microflagellate Cafeteria reonbergensis". Applied and Environmental Microbiology. 63 (2): 749–752. Bibcode:1997ApEnM..63..749O. doi:10.1128/AEM.63.2.749-752.1997. PMC 1389530. PMID 16535524.
- Massana, Ramon; Javier Del Campo; Christian Dinter; Ruben Sommaruga (2007). "Crash of a population of the marine heterotrophic flagellate Cafeteria roenbergensis by viral infection". Environmental Microbiology. 9 (11): 2660–2669. doi:10.1111/j.1462-2920.2007.01378.x. PMID 17922751. S2CID 30191542.
- Guiry, M. D.; Guiry, G. M. (2012). "AlgaeBase". National University of Ireland, Galway. Retrieved 16 January 2012.
- Cavalier-Smith, T., Chatton, Moestrup, T. Fenchel, D. J. Patterson, and Larsen. "Taxonomy Browser : Algaebase." Algaebase :: Listing the World's Algae. Algae Base. Web. 16 Jan. 2012. <http://www.algaebase.org/browse/taxonomy/?id=6698>.
- Munn, C. B. (2011). Marine Microbiology: Ecology and Applications. New York: Garland Science.
- Thomas Ishigaki; M. A. Sleigh (2001). "Grazing characteristics and growth efficiencies at two different temperatures for three nanoflagellates fed with Vibrio bacteria at three different concentrations". Microbial Ecology. 41 (3): 264–271. doi:10.1007/s002480000060. JSTOR 4251819. PMID 11391464. S2CID 29625660.
- Fischer, M. G.; Allen, M. J.; Wilson, W. H.; Suttle, C. A. (2010). "Giant virus with a remarkable complement of genes infects marine zooplankton" (PDF). Proceedings of the National Academy of Sciences. 107 (45): 19508–19513. Bibcode:2010PNAS..10719508F. doi:10.1073/pnas.1007615107. PMC 2984142. PMID 20974979.
- Fischer MG, Suttle CA (April 2011). "A virophage at the origin of large DNA transposons". Science. 332 (6026): 231–4. Bibcode:2011Sci...332..231F. doi:10.1126/science.1199412. PMID 21385722. S2CID 206530677.
- Fischer MG, Hackl (December 2016). "Host genome integration and giant virus-induced reactivation of the virophage mavirus" (PDF). Nature. 540 (7632): 288–91. Bibcode:2016Natur.540..288F. doi:10.1038/nature20593. PMID 27929021. S2CID 4458402.
- Fenchel, T. (1988). "Marine plankton food chains". Annual Review of Ecology and Systematics. 19: 19–38. doi:10.1146/annurev.es.19.110188.000315.
- Patterson, David J. "Cafeteria roenbergensis." Encyclopedia of Life. Web. 9 Jan. 2012. <http://eol.org/pages/912371/overview>
- O'Kelly, C. J., B. F. Lang, and G. Burger. "Cafeteria roenbergensis –Mitochondrial Genome Organization ..." Evolutionary & Integrative Genomics. University of Montreal. Web. 16 Jan. 2012. <http://megasun.bch.umontreal.ca/ogmp/projects/croen/gen.html>.
- Park, J. S.; Simpson, A. G. B. (2010). "Characterization of halotolerant Bicosoecida and Placididea (Stramenopila) that are distinct from marine forms, and the phylogenetic pattern of salinity preference in heterotrophic stramenopiles". Environmental Microbiology. 12 (5): 1173–1184. doi:10.1111/j.1462-2920.2010.02158.x. PMID 20132281. S2CID 20449399.