Cefovecin
Cefovecin (INN) is an antibiotic of the cephalosporin class, licensed for the treatment of skin infections in cats and dogs. It is marketed by Zoetis under the trade name Convenia. It is used to treat skin infections caused by Pasteurella multocida in cats, and Staphylococcus intermedius and Streptococcus canis in dogs. The advantage of using a long-acting injectable antibiotic is that, unlike with daily administration, doses cannot be missed, which may allow partially resistant microbes to recover. The disadvantage is the presence of subtherapeutic concentrations in the weeks after the resolution of infections. This is associated with the development of resistance in microbes. It should not be used in pregnant or lactating animals or in animals with a history of allergies to penicillin or cephalosporin drugs.[1]
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Pregnancy category |
|
| Routes of administration | dorsoscapular subcutaneous injection |
| ATCvet code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | Dogs: 98.5%, Cats: 99.8% |
| Metabolism | none |
| Elimination half-life | Dogs: 133 hours, Cats: 166 hours |
| Excretion | unchanged in urine/bile |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
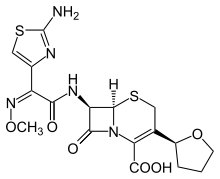
| Formula | C17H19N5O6S2 |
| Molar mass | 453.49 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Medical uses
Cefovecin is a broad-spectrum, third-generation cephalosporin antibiotic administered by subcutaneous injection.[2] It is used to treat skin and soft tissue infections in dogs and cats.[2] The antimicrobial effects last for 14 days following administration.[3]
In drug studies, cefovecin administered to dogs was 92.4% effective against skin infections (secondary superficial pyoderma, abscesses, and infected wounds). In cats, it was 96.8% effective against skin infections.[1]
Contraindications
Contraindications include known allergies to cefovecin or antibiotics containing β-lactam rings such as penicillin or cephalosporins. Adverse reactions can include anaphylaxis. It is not for use in humans and should be kept out of reach of children. People with similar known allergies should avoid dermal contact when handling cefovecin.
Adverse effects
In dogs, adverse effects may include lethargy, decreased appetite, vomiting, diarrhea, blood in feces, and flatulence. In cats, adverse reactions may include vomiting, diarrhea, decreased appetite, lethargy, odd hyperactive behavior, and inappropriate urination. Mildly increased serum alanine transaminase (ALT) and gamma-glutamyltransferase may also occur.[1]
Other reported events in dogs and cats include death, tremors/ataxia, seizures, anaphylaxis, acute pulmonary edema, facial edema, injection site reactions (alopecia, scabs, necrosis, and erythema), hemolytic anemia, salivation, pruritus, lethargy, vomiting, diarrhea, and inappetence.[4]
Pharmacology
Mechanism of action
Cefovecin interferes with the synthesis of bacterial cell walls, by binding to penicillin binding proteins. Due to high protein-binding, it is not effective against species of Pseudomonas or Enterococcus. The maximum anti-bacterial activity occurs approximately two days after cefovecin has been administered.[5]
Society and culture
Cefovecin was first authorized for use in the European Union in June 2006,[7] and was approved for use in the United States in June 2008.[8]
References
- Pfizer Animal Health. "Pfizer Convenia" (PDF). Pfizer. Retrieved 29 January 2013.
- Pfizer Animal Health. "Convenia". Pfizer. Archived from the original on 11 April 2012. Retrieved 29 January 2013.
- Maddison JE, Page SW, Church DB, eds. (2008). "Chapter 8: Antibacteria drugs. Cephalosporins and cephamycins". Small animal clinical pharmacology (2nd ed.). Edinburgh: Saunders/Elsevier. pp. 164–168. ISBN 9780702028588.
- Convenia Prescribing Information. "Convenia Prescribing Information" (PDF). Pfizer. Retrieved 18 October 2015.
- Boothe DM (2012). "Chapter 7: Antimicrobial drugs". In Boothe DM (ed.). Small animal clinical pharmacology & therapeutics (2nd ed.). St. Louis, Mo.: Elsevier Saunders. ISBN 9781437723571.
- Thuesen LR, Bertelsen MF, Brimer L, Skaanild MT (December 2009). "Selected pharmacokinetic parameters for Cefovecin in hens and green iguanas" (PDF). Journal of Veterinary Pharmacology and Therapeutics. 32 (6): 613–7. doi:10.1111/j.1365-2885.2009.01083.x. PMID 20444017.
- "European Public Assessment Report for Convenia (from the EMEA website)". Archived from the original on 2008-08-04. Retrieved 2008-07-11.
- "FDA Approves First and Only Single-dose Antibiotic for Dogs and Cats" (Press release). Pfizer Inc. 2008-06-30. Retrieved 2008-07-11.
External links
- CID 23670874 from PubChem - Cefovecin sodium