Decompressive craniectomy
Decompressive craniectomy (crani- + -ectomy) is a neurosurgical procedure in which part of the skull is removed to allow a swelling brain room to expand without being squeezed. It is performed on victims of traumatic brain injury, stroke, Chiari Malformation, and other conditions associated with raised intracranial pressure. Use of the surgery is controversial.[1]
| Decompressive craniectomy | |
|---|---|
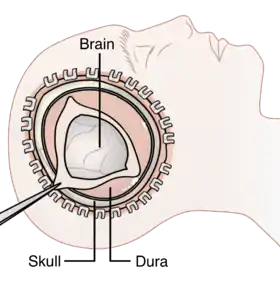 Diagram showing the elements of a decompressive craniectomy | |
| ICD-9-CM | 01.2, 02.02 |
| MeSH | D056424 |
The procedure evolved from a primitive form of surgery known as trephining or trepanning. The older procedure, while common in prehistoric times, was deprecated in favor of other, less invasive treatments as they were developed; although it was still performed with some frequency prior to the twentieth century, its resurgence in modern form became possible only upon the development of precision cutting tools, cranial drills, and sophisticated post-operative care such as antibiotics.
Results of clinical trials
Reduction of intracranial pressure
Though the procedure is considered a last resort, some evidence suggests that it does improve outcomes by lowering intracranial pressure (ICP), the pressure within the skull.[1][2][3] Raised intracranial pressure is very often debilitating or fatal because it causes compression of the brain and restricts cerebral blood flow. The aim of decompressive craniectomy is to reduce this pressure. The part of the skull that is removed is called a bone flap. A study has shown that the larger the removed bone flap is, the more ICP is reduced.[4]
DECRA trial
In March 2011, investigators from Australia and several other countries published the results of the DECRA[5] trial in The New England Journal of Medicine. This was a randomized trial comparing decompressive craniectomy to best medical therapy run between 2002 and 2010 to assess the optimal management of patients with medically refractory ICP following diffuse non-penetrating head injury. The study investigators found that decompressive craniectomy was associated with worse functional outcomes, as measured by a standard metric, than best medical care. There were no differences in deaths between groups. However, the results of the DECRA trial have been rejected or at least questioned by many practicing neurosurgeons, and a concurrently published editorial raises several study weaknesses.[6] First, the threshold for defining increased ICP, and the time allowed before declaring ICP medically refractory, are not what many practicing physicians would consider increased or refractory. Second, out of almost 3500 potentially eligible patients, only 155 patients were enrolled, showing that the study cannot be generalized to all patients with severe non-penetrating brain injury.[7] Lastly, more subjects in the craniectomy group had unreactive pupils than patients in the medical therapy group after randomisation and before surgical intervention; thus making this a possible confounding factor.[8]
Other effects
In addition to reducing ICP, studies have found decompressive craniectomy to improve cerebral perfusion pressure[1][3] and cerebral blood flow in head injured patients.[1]
Decompressive craniectomy is also used to manage major strokes, associated with "malignant" edema and intracranial hypertension. The pooled evidence from three randomised controlled trials in Europe supports the retrospective observations that early (within 48 hours) application of decompressive craniectomy after "malignant" stroke may result in improved survival and functional outcome in patients under the age of 55, compared to conservative management alone.[9]
The procedure is recommended especially for young patients in whom ICP is not controllable by other methods.[1] Age of greater than 50 years is associated with a poorer outcome after the surgery.[3]
Complications
Infections such as meningitis or brain abscess can occur after decompressive craniectomy.[10]
Children
In severely head injured children, a study has shown that decompressive craniectomy resulted in good recovery in all children in the study, suggesting the procedure has an advantage over non-surgical treatment in children.[11] In one of the largest studies on pediatric patients, Jagannathan et al. found a net 65% favorable outcomes rate in pediatric patients for accidental trauma after craniectomy when followed for more than five years. Only three patients were dependent on caregivers.[12] This is the only prospective randomly controlled study to date to support the potential benefit of decompressive craniectomy following traumatic brain injury.[13]
Follow-up treatment
After a craniectomy, the risk of brain injury is increased, particularly after the patient heals and becomes mobile again. Therefore, special measures must be taken to protect the brain, such as a helmet or a temporary implant in the skull.[14]
When the patient has healed sufficiently, the opening in the skull is usually closed with a cranioplasty. If possible, the original skull fragment is preserved after the craniectomy in anticipation of the cranioplasty.[15]
Ongoing trials
The RESCUEicp study is an international multicenter trial that finished recruitment in March 2014. The aim of this study is to determine the effectiveness of decompressive craniectomy, compared to medical management alone, to treat brain swelling and improve outcome. This study is coordinated by the University of Cambridge Academic Neurosurgery Unit[16] and the European Brain Injury Consortium (EBIC).[17]
The RESCUE-ASDH study Official RESCUE-ASDH Trial Site is a multicenter, pragmatic, parallel group randomised trial that aims to compare the clinical and cost-effectiveness of decompressive craniectomy versus craniotomy for the management of adult head-injured patients undergoing evacuation of an acute subdural haematoma (ASDH). The trial has started recruiting, and is expected to run until 2020. This study is coordinated by the University of Cambridge Academic Neurosurgery Unit
References
- Kunze, E; Meixensberger J; Janka M; Sorensen N; Roosen K (1998). "Decompressive craniectomy in patients with uncontrollable intracranial hypertension". Acta Neurochirurgica. Supplement. 71: 16–18. doi:10.1007/978-3-7091-6475-4_5. ISBN 978-3-7091-7331-2. PMID 9779131.
- Aarabi, B; Hesdorffer DC; Ahn ES; Aresco C; Scalea TM; Eisenberg HM (2006). "Outcome following decompressive craniectomy for malignant swelling due to severe head injury". Journal of Neurosurgery. 104 (4): 469–479. doi:10.3171/jns.2006.104.4.469. PMID 16619648. S2CID 22490737.
- Schneider, GH; Bardt T; Lanksch WR; Unterberg A (2002). "Decompressive craniectomy following traumatic brain injury: ICP, CPP and neurological outcome". Acta Neurochirurgica. Supplement. 81: 77–79. doi:10.1007/978-3-7091-6738-0_20. ISBN 978-3-7091-7397-8. PMID 12168363. S2CID 38854921.
- Skoglund, TS; Eriksson-Ritzen C; Jensen C; Rydenhag B (2006). "Aspects on decompressive craniectomy in patients with traumatic head injuries". Journal of Neurotrauma. 23 (10): 1502–9. doi:10.1089/neu.2006.23.1502. PMID 17020484.
- Cooper, DJ; et al. (25 March 2011). "Decompressive craniectomy in diffuse traumatic brain injury" (PDF). New England Journal of Medicine. 364 (16): 1493–502. doi:10.1056/NEJMoa1102077. PMID 21434843.
- Servadei, F (2011). "Decompressive craniectomy in diffuse traumatic brain injury" (PDF). New England Journal of Medicine. 364 (16): 1493–502. doi:10.1056/NEJMoa1102077. PMID 21434843.
- Servadei F (April 2011). "Clinical value of decompressive craniectomy". The New England Journal of Medicine. 364 (16): 1558–9. doi:10.1056/NEJMe1102998. PMID 21434844.
- García Vicente E, Garnelo Rey V, Manikon M, Ashworth S, Wilson MH (2013). "Does Early Decompressive Craniectomy Improve Outcome? Experience from an Active UK Recruiter Centre". Case Reports in Critical Care. 2013: 714945. doi:10.1155/2013/714945. PMC 4010016. PMID 24829829.
- Vahedi K, Hofmeijer J, Juettler E, et al. (2007). "Early decompressive surgery in malignant infarction of the middle cerebral artery: a pooled analysis of three randomised controlled trials". Lancet Neurology. 6 (3): 215–22. doi:10.1016/S1474-4422(07)70036-4. PMID 17303527. S2CID 23509663.
- Albanese, J; Leone M; Alliez JR; Kaya JM; Antonini F; Alliez B; Martin C (2003). "Decompressive craniectomy for severe traumatic brain injury: Evaluation of the effects at one year". Critical Care Medicine. 31 (10): 2535–2538. doi:10.1097/01.CCM.0000089927.67396.F3. PMID 14530763. S2CID 44700600.
- Hejazi, N; Witzmann A; Fae P (February 2002). "Unilateral decompressive craniectomy for children with severe brain injury. Report of seven cases and review of the relevant literature". European Journal of Pediatrics. 161 (2): 99–104. doi:10.1007/s00431-001-0864-x. PMID 11954760. S2CID 10175707.
- Jagannathan, J; Okonkwo DO; Dumont, AS (April 2007). "Outcome following decompressive craniectomy in children with severe traumatic brain injury: a 10-year single-center experience with long-term follow up". Journal of Neurosurgery: Pediatrics. 106 (4): 268–275. doi:10.3171/ped.2007.106.4.268. PMID 17465359.
- Sahuquillo J, Arikan F (2006). Sahuquillo, Juan (ed.). "Decompressive craniectomy for the treatment of refractory high intracranial pressure in traumatic brain injury". Cochrane Database of Systematic Reviews (1): CD003983. doi:10.1002/14651858.CD003983.pub2. PMID 16437469.
- S. Boström; L. Bobinski; P. Zsigmond; A. Theodorsson (2005). "Improved brain protection at decompressive craniectomy – a new method using Palacos R-40 (methylmethacrylate)". Acta Neurochirurgica. 147 (3): 279–281. doi:10.1007/s00701-004-0480-4. PMID 15662564. S2CID 22544660.
- N. Grossman; H. S. Shemesh-Jan; V. Merkin; M. Gideon; A. Cohen (2007). "Deep-freeze preservation of cranial bones for future cranioplasty: nine years of experience in Soroka University Medical Center". Cell and Tissue Banking. 8 (3): 243–246. doi:10.1007/s10561-006-9032-x. PMID 17273898. S2CID 6333710.
- University of Cambridge Academic Neurosurgery Unit
- EBIC.nl - European Brain Injury Consortium