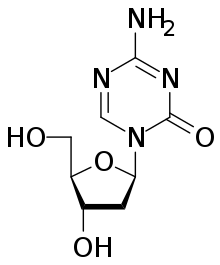
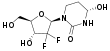
Decitabine/cedazuridine
Decitabine/cedazuridine, sold under the brand name Inqovi, is a fixed-dose combination medication for the treatment of adults with myelodysplastic syndromes (MDS) and chronic myelomonocytic leukemia (CMML).[6][7][8] It is a combination of decitabine, a nucleoside metabolic inhibitor, and cedazuridine, a cytidine deaminase inhibitor.[6][7][8][9][10]
| |||
| Combination of | |||
|---|---|---|---|
| Decitabine | Nucleoside metabolic inhibitor | ||
| Cedazuridine | Cytidine deaminase inhibitor | ||
| Clinical data | |||
| Trade names | Inqovi | ||
| Other names | ASTX727, C-DEC | ||
| AHFS/Drugs.com | Monograph | ||
| License data | |||
| Pregnancy category |
| ||
| Routes of administration | By mouth | ||
| ATC code |
| ||
| Legal status | |||
| Legal status | |||
| Identifiers | |||
| CAS Number | |||
| KEGG | |||
The most common side effects of decitabine/cedazuridine include fatigue, constipation, hemorrhage, muscle pain (myalgia), mucositis (mouth sores), arthralgia (joint pain), nausea, dyspnea, diarrhea, rash, dizziness, fever with low white blood cell count (febrile neutropenia), edema, headache, cough, decreased appetite, upper respiratory tract infection, pneumonia, and transaminase increased.[6][5] The combination can cause fetal harm.[6][5][11]
Decitabine/cedazuridine was approved for medical use in the United States and in Canada in July 2020.[6][7][8][11]
Medical uses
Decitabine/cedazuridine is indicated for treatment of adults with myelodysplastic syndromes (MDS), including previously treated and untreated, de novo and secondary MDS with the following French American-British subtypes (refractory anemia, refractory anemia with ringed sideroblasts, refractory anemia with excess blasts, and chronic myelomonocytic leukemia [CMML]) and intermediate-1, intermediate-2, and high-risk International Prognostic Scoring System groups.[6][7][5]
MDS is a type of blood cancer in which blood cells in the bone marrow are defective leading to a low number of one or more types of blood cells.[11]
History
Decitabine/cedazuridine was approved for medical use in the United States and in Canada in July 2020.[6][7][8][11]
The U.S. Food and Drug Administration (FDA) granted the application of decitabine combined with cedazuridine priority review and orphan drug designation.[6][12] The orphan drug designation was granted in August 2019 for the treatment of myelodysplastic syndromes (including chronic myelomonocytic leukemia).[12][13]
Decitabine combined with cedazuridine was investigated in two open-label, randomized, crossover trials.[7] Trial ASTX727-01-B (NCT02103478) included 80 adult participants with MDS (International Prognostic Scoring System [IPSS] Intermediate-1, Intermediate-2, or high-risk) or CMML and trial ASTX727-02 (NCT03306264) included 133 adult participants with MDS or CMML, including all French-American-British classification criteria and IPSS Intermediate-1, Intermediate-2, or high-risk prognostic scores.[7] The trials were conducted at 51 sites in the United States and Canada.[11]
In both trials, participants were randomized 1:1 to receive decitabine combined with cedazuridine (35 mg decitabine and 100 mg cedazuridine) orally in cycle 1 and decitabine 20 mg/m2 intravenously in cycle 2 or the reverse sequence.[7] Both decitabine combined with cedazuridine and intravenous decitabine were administered once daily on days 1 through 5 of a 28-day cycle.[7] Starting with cycle 3, all participants received decitabine combined with cedazuridine orally once daily on days 1 through 5 of each 28-day cycle until disease progression or unacceptable toxicity.[7] Both trials provided comparison of exposure and safety in the first two cycles between oral decitabine combined with cedazuridine and intravenous decitabine and description of disease response with decitabine combined with cedazuridine.[7] Comparison of disease response between the decitabine combined with cedazuridine and intravenous decitabine was not possible because all participants received decitabine combined with cedazuridine starting from Cycle 3.[7]
The FDA approval of decitabine combined with cedazuridine was based on clinical trial results which showed similar drug concentrations between intravenous decitabine and decitabine combined with cedazuridine.[6][7] Additionally, about half of the participants who were formerly dependent on transfusions were able to no longer require transfusions during an 8-week period.[6] The safety profile of decitabine combined with cedazuridine was also similar to intravenous decitabine.[6] The FDA granted the approval of Inqovi to Astex Pharmaceuticals, Inc., a subsidiary of Otsuka Pharmaceutical Co. Ltd.[6]
References
- "Australian Public Assessment Report for Decitabine and cedazuridine" (PDF). Therapeutic Goods Administration. The Australian Government. January 2021.
- Otsuka Australia Pharmaceutical Pty Ltd (22 September 2020). "Australian Product Information – Inqovi 35/100 (Decitabine and Cedazuridine ) Tablets" (PDF). Therapeutic Goods Administration. The Australian Government.
- "Public Record: INQOVI 35/100 decitabine 35 mg and cedazuridine 100 mg tablet bottle". Therapeutic Goods Administration. The Australian Government. Archived from the original on 28 August 2021. Retrieved 7 February 2022.
- "Summary Basis of Decision (SBD) for Inqovi". Health Canada. Retrieved 29 May 2022.
- "Inqovi (decitabine and cedazuridine) tablets, for oral use" (PDF). U.S. Food and Drug Administration (FDA). Otsuka Pharmaceutical Co. Retrieved 7 July 2020.
- "FDA Approves New Therapy for Myelodysplastic Syndromes (MDS) That Can Be Taken at Home". U.S. Food and Drug Administration (FDA) (Press release). 7 July 2020. Retrieved 7 July 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - "FDA approves oral combination of decitabine and cedazuridine for myelodysplastic syndromes". U.S. Food and Drug Administration (FDA). 7 July 2020. Retrieved 7 July 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - "Astex Pharmaceuticals, Taiho Oncology, and Otsuka Pharmaceutical announce FDA and Health Canada approval of Inqovi (decitabine and cedazuridine) tablets, oral hypomethylating agent (HMA) therapy for intermediate and high-risk MDS and CMML". Taiho Pharmaceutical Group (Press release). 7 July 2020. Retrieved 19 July 2020.
- Dhillon S (September 2020). "Decitabine/Cedazuridine: First Approval". Drugs. 80 (13): 1373–1378. doi:10.1007/s40265-020-01389-7. PMC 7708383. PMID 32860582.
- Thota S, Oganesian A, Azab M, Griffiths EA (June 2021). "Role of cedazuridine/decitabine in the management of myelodysplastic syndrome and chronic myelomonocytic leukemia". Future Oncology. London, England. 17 (16): 2077–2087. doi:10.2217/fon-2020-1210. PMID 33709786. S2CID 232206560.
- "Drug Trials Snapshots: Inqovi". U.S. Food and Drug Administration (FDA). 7 July 2020. Retrieved 25 July 2020.
- "Inqovi Orphan Drug Designations and Approvals". U.S. Food and Drug Administration (FDA). 21 August 2019. Retrieved 7 July 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - "Astex Pharmaceuticals announces U.S. Food and Drug Administration (FDA) acceptance for review of an NDA for the combination oral hypomethylating agent cedazuridine and decitabine (ASTX727 or oral C-DEC), for the treatment of MDS and CMML". Astex (Press release). 11 February 2020. Retrieved 7 July 2020.
External links
- "Decitabine". Drug Information Portal. U.S. National Library of Medicine.
- "Cedazuridine". Drug Information Portal. U.S. National Library of Medicine.
- "Decitabine". National Cancer Institute.
- "Cedazuridine". National Cancer Institute.
- Clinical trial number NCT02103478 for "Pharmacokinetic Guided Dose Escalation and Dose Confirmation With Oral Decitabine and Oral CDAi in Patients With MDS" at ClinicalTrials.gov
- Clinical trial number NCT03306264 for "Study of ASTX727 vs IV Decitabine in MDS, CMML, and AML" at ClinicalTrials.gov
- "ASTX727 – Hematologic Malignancies". Astex.