Dexpramipexole
Dexpramipexole (KNS-760704) is an orally administered drug candidate shown to selectively and significantly lower eosinophil counts in human blood and tissue.[1] The drug is currently in clinical development for eosinophil-associated diseases[2] by Knopp Biosciences LLC.
 | |
| Names | |
|---|---|
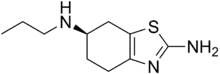
| Preferred IUPAC name
(6R)-N6-Propyl-4,5,6,7-tetrahydro-1,3-benzothiazole-2,6-diamine | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID |
|
| UNII |
|
CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C10H17N3S |
| Molar mass | 211.33 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
In a Phase 2 clinical trial (AS201), dexpramipexole significantly lowered eosinophil counts and improved lung function in subjects with moderate-to-severe eosinophilic asthma.[3] Previous clinical trials showed that dexpramipexole significantly reduced eosinophil counts and glucocorticoid requirements in patients with hypereosinophilic syndrome (HES)[4] and significantly reduced blood and tissue eosinophil counts in patients with chronic rhinosinusitis with nasal polyps.[5] The sponsor is continuing development of dexpramipexole in eosinophilic asthma, HES, and eosinophilic gastrointestinal diseases.
The drug was originally investigated by Knopp Biosciences and Biogen Idec for the treatment of amyotrophic lateral sclerosis (ALS), also known as Lou Gehrig’s disease. A 2010 Phase II clinical trial showed a slowing of ALS disease progression and mortality benefits.[6] In January 2013, Biogen Idec announced it was discontinuing its development of dexpramipexole in ALS due to lack of efficacy in a Phase III study.[7]
Dexpramipexole is the enantiomer of pramipexole. It is a low molecular weight, orally bioavailable, water-soluble small molecule with linear pharmacokinetics.[8] Dexpramipexole was originally identified as a candidate therapy for ALS by James Bennett, M.D., Ph.D., then of the University of Virginia.
See also
- Pramipexole, a dopamine agonist, is the enantiopure (S)-isomer of dexpramipexole. Dexpramipexole has essentially no dopamine agonist activity.[8]
References
- Dworetzky, SI; et al. (March 2017). "The targeted eosinophil-lowering effects of dexpramipexole in clinical studies". Blood Cells Mol. Dis. 2017 Mar: 63:62–65. doi:10.1016/j.bcmd.2017.01.008. PMID 28178599.
- Gleich, Gerald (August 2018). "Dexpramipexole: a new antieosinophil drug?". Blood. 132 (5): 461–462. doi:10.1182/blood-2018-06-851600. PMID 30072411.
- Prussin, Calman; et al. (May 17, 2021). "Oral Dexpramipexole Efficacy in Lowering Blood Eosinophils in Patients with Moderate to Severe Uncontrolled Eosinophilic Asthma" (PDF). American Thoracic Society 2021 International Conference.
- Panch, Sandhya; et al. (August 2, 2018). "Dexpramipexole as an oral steroid-sparing agent in hypereosinophilic syndromes". Blood. 2018 Aug 2, 132(5) (5): 501–509. doi:10.1182/blood-2018-02-835330. PMC 6073324. PMID 29739754.
- Laidlaw, Tanya; et al. (February 2019). "Dexpramipexole depletes blood and tissue eosinophils in nasal polyps with no change in polyp size". Laryngoscope. 129 (2): E61–E66. doi:10.1002/lary.27564. PMID 30284267. S2CID 52915205.
- Cudkowicz, Merit; et al. (November 20, 2011). "The effects of dexpramipexole (KNS-760704) in individuals with amyotrophic lateral sclerosis". Nature Medicine. 17 (12): 1652–1656. doi:10.1038/nm.2579. PMID 22101764. S2CID 205388563.
{{cite journal}}: CS1 maint: date and year (link) - "Biogen Idec Reports Top-Line Results from Phase 3 Trial Investigating Dexpramipexole in People with Amyotrophic Lateral Sclerosis (ALS)". Biogen Idec. Archived from the original on 22 January 2013. Retrieved 4 January 2013.
- Gribkoff, Valentin; Bozik, Michael (August 13, 2008). "KNS‐760704 [(6R)‐4,5,6,7‐tetrahydro‐N6‐propyl‐2, 6‐benzothiazole‐diamine dihydrochloride monohydrate] for the Treatment of Amyotrophic Lateral Sclerosis". CNS Neurosci. Ther. 14 (3): 215–226. doi:10.1111/j.1755-5949.2008.00048.x. PMC 6494033. PMID 18801114.
External links
- STATEMENT ON A NONPROPRIETARY NAME ADOPTED BY THE USAN COUNCIL, contains structural formula and chemical name
- Clinicaltrials.gov: Dexpramipexole Dose-Ranging Biomarker Study in Subjects With Eosinophilic Asthma (AS201)
- Clinicaltrials.gov:Study to Evaluate Safety and Efficacy of Dexpramipexole (KNS-760704) in Subjects With Hypereosinophilic Syndrome
- Clinicaltrials.gov:Study of Dexpramipexole Chronic Sinusitis With Nasal Polyps and Eosinophilia (CS201)