Diffuse optical mammography
Diffuse optical mammography, or simply optical mammography, is an emerging imaging technique that enables the investigation of the breast composition through spectral analysis. It combines in a single non-invasive tool the capability to implement breast cancer risk assessment,[2] lesion characterization,[3] therapy monitoring[4] and prediction of therapy outcome.[5] It is an application of diffuse optics, which studies light propagation in strongly diffusive media, such as biological tissues, working in the red and near-infrared spectral range, between 600 and 1100 nm.[6]
| Diffuse optical mammography | |
|---|---|
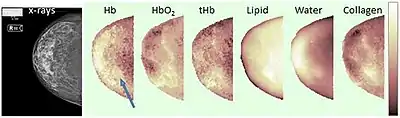 Example of breast constituents' concentrations maps through optical mammography (right cranio-caudal view). The blue arrow points to the lesion. Hb stands for deoxy-hemoglobin, HbO2 for oxy-hemoglobin, tHb for total hemoglobin.[1] | |
| Purpose | investigation of the breast composition through spectral analysis |
Comparison with conventional imaging techniques
Currently, the most common breast imaging techniques are X-ray mammography, ultrasounds, MRI and PET.
X-ray mammography is widely spread for breast screening, thanks to its high spatial resolution[7] and the short measurement time. However, it is not sensitive to the breast physiology,[8] it is characterized by a limited efficiency in investigating dense breasts[9] and it is harmful due to the use of ionizing radiation.[10] Ultrasounds are non-invasive and they are used especially on young women,[11] who are usually characterized by dense breasts, but the images interpretation depends on the operator's experience. MRI shows a good correlation with the tumour dimensions and is claimed to be the best method for the identification and characterization of lesions.[12] Even though there is no verified long-term health risk from the magnetic fields employed during an MRI, it is not used as first investigative tool because of the high costs and the elevated duration of the exam.[13] Finally, PET allows the early evaluation of the metabolic changes of the tumour,[14] but it is very expensive and requires the administration of a radioactive tracer. For this reason, its application is not frequently recommended.
On the contrary, optical mammography is cheap, efficient also on dense breasts, and devoid of any side effect, so that it can be used to track the evolution of the patient's condition on a daily basis. It is also able to characterize breast from a physiologic point of view.[15] However, being still under development, there is a lack of standardization in data analysis among the research groups dealing with it, and it suffers from low spatial resolution. For this reason, a "multimodal approach" is suggested, where optical mammography is complementary to another conventional technique, so that also the diagnostic efficacy is improved.[10][15]
Physical mechanism
Photon migration in diffusive media
Biological tissues are diffusive media, which means that light attenuation during propagation is due not only to absorption, but also to scattering. The former is related to the chemical composition of the medium and induces photon annihilation, whereas the latter depends on the microscopic inhomogeneities of its refractive index and determines deviations in photon's trajectory.[6] The absorption coefficient represents the probability per unit length that an absorption event takes place, while the scattering coefficient denotes the probability per unit length that a scattering event occurs.[16] However, many studies refer to the reduced scattering coefficient rather than the simple scattering coefficient, in order to take into account the medium's anisotropy. The medium's anisotropy is represented by the factor , which is the average cosine of the angular deflection.[6]
Light propagation through highly diffusive media is typically described through the heuristic approach of the radiative transport theory, sided by the so-called “diffusion approximation”: scattering is assumed to be isotropic and strongly dominant over absorption. This is fairly accurate for example for the breast tissue, in the red and near infrared spectral range (between 600 and 1100 nm), known also as "therapeutic window". In the therapeutic window, light can penetrate a few centimetres, so that it can explore the volume at exam. This is the reason why photon migration in biological tissues is known also as "diffuse optics".[6]
The relation between reduced scattering coefficient and wavelength () derives from the Mie theory:[17]

where is the reference wavelength and and refer to the size of the scattering centres and their density, respectively.
Regarding the absorption coefficient, the relation with is mediated by the so-called “extinction coefficient” ,[18] that in combination with the Lambert-Beer law gives
where is the concentration of the ith breast constituent. Measuring at different wavelengths, the breast constituents’ concentrations can be extrapolated.
Breast constituents' absorption spectra
The main breast constituents are oxy and deoxy-hemoglobin, water, lipids and collagen.[1] In particular, collagen has been recognized as an independent risk factor for developing breast cancer.[19]
Blood strongly absorbs in the red spectral range, whereas collagen, water and lipids have their absorption peaks at wavelengths longer than 900 nm. The distinction between oxy and deoxy-haemoglobin is due to the presence of a second large peak in the case of oxy-haemoglobin. Lipids are characterized by absorption maxima at 930 nm and 1040 nm, while the wavelength 975 nm is sensitive to water. Finally, an absorption peak for collagen takes place at 1030 nm.[16][1]
Possible implementations
Diffuse optical mammography can be implemented exploiting three different approaches: time domain,[20] frequency domain[21] and continuous wave.[22] Moreover, there exist two main geometries to perform an optical measurement:
- Reflectance: injection and collection occur on the same side of the breast. The woman is usually prone or bent forward and places the breast on a support provided with a hole where sources and detectors are located.[23] Other systems' setups instead require the woman to lie supine and the measurement is carried out with a hand-held probe.[24]
- Transmittance: injection and collection occur on opposite sides of the breast. The breast is usually compressed between plane parallel plates.[25][26]
Whatever the chosen approach is, any optical mammograph must comprehend some essential elements: laser sources, a detector, a signal processor.
The use of multiple laser sources allows to investigate the breast constituents' concentrations of interest, by selecting some specific wavelengths. Detectors are usually photomultiplier tubes[23] or avalanche photodiodes.[27] Finally, the signal processor could be a device for Time-correlated single photon counting[28] in the case of a time-resolved optical mammograph,[25] or a filter for frequency modulation in the case of frequency-domain ones.[29]
Based on the number and position of sources and detectors, an optical mammograph can produce bidimensional or three-dimensional breast constituents' maps.
Time domain
In time-domain measurements, short light pulses of the order of hundreds of picoseconds are delivered to the breast and its optical properties are retrieved from the features of the re-emitted pulses, which have undergone delay, broadening and attenuation.[25][30] Time-correlated single photon counting is fundamental to cope with the low-level output signal.[28]
Frequency domain
In frequency-domain measurements, an intensity-modulated signal is injected into the breast and its optical properties are deduced from the dephasement and the demodulation of the output signal with respect to the input one. The measurement is repeated for different values of the frequency modulation.[29][31]
Continuous wave
In continuous wave (CW) measurements, the light source is a continuous wave laser, which hinders the separation of the absorption and scattering contributions with a single measurement. A possible solution is to perform space or angle-resolved measurements. In general, the CW approach is combined with the frequency domain one, in order to reinforce the strengths of both.[27]
Potential applications
Breast cancer risk assessment
A denser breast is more likely to develop breast cancer.[19] A dense breast is characterized by a meaningful amount of fibrous tissue, relatively to the adipose one. The main constituents of a fibrous tissue are water, collagen and hemoglobin and optical mammography is able to discriminate and quantify tissues' components.[2] Therefore, by measuring breast constituents' concentrations, optical mammography could assess breast cancer risk.[2][32][33]
Lesion characterization
Tumours are generally made of fibrous tissue and could be recognized in the constituents' maps as local spots with higher concentrations of water, collagen and hemoglobin with respect to the surrounding, mostly adipose, healthy tissues. Studies demonstrate that the variation in concentration with respect to the healthy tissue is statistically more marked in the case of malignant tumours than benign ones.[34][35] In addition, the scattering coefficient is generally higher for benign lesions. Such distinctions suggest that optical mammography could characterize breast lesions.[34][35][36][37]
Therapy monitoring and prediction of therapy outcome
Breast cancer management depends on the characteristics of the tumour and the patient's condition. One of the possible strategies is the administration of neoadjuvant therapy, whose goal is to shrink the tumour size before surgery.[38] Studies show that if the therapy is efficient, then the water, collagen and hemoglobin contents of the lesion show a decreasing behaviour over time, which suggests that the initially fibrous tissue acquires features similar to the adipose one.[4][39] Optical measurements in correspondence with therapy sessions could track its evolution, so to assess the patient's response to it. Moreover, it is believed that therapy effectiveness could be predicted even on the first day of treatment on the base of initial breast constituents' concentrations.[40][5]
See also
- Breast imaging
- Diffuse optical imaging
- Optical tomography
- Radiative transfer equation and diffusion theory for photon transport in biological tissue
- Near-infrared window in biological tissue
- Time-domain diffuse optics
- Computed tomography laser mammography
References
- Taroni, Paola; Paganoni, Anna Maria; Ieva, Francesca; Pifferi, Antonio; Quarto, Giovanna; Abbate, Francesca; Cassano, Enrico; Cubeddu, Rinaldo (16 January 2017). "Non-invasive optical estimate of tissue composition to differentiate malignant from benign breast lesions: A pilot study". Scientific Reports. 7 (1): 40683. Bibcode:2017NatSR...740683T. doi:10.1038/srep40683. PMC 5238417. PMID 28091596. S2CID 33523292.
- Taroni, Paola; Pifferi, Antonio; Quarto, Giovanna; Spinelli, Lorenzo; Torricelli, Alessandro; Abbate, Francesca; Villa, Anna; Balestreri, Nicola; Menna, Simona; Cassano, Enrico; Cubeddu, Rinaldo (2010). "Noninvasive assessment of breast cancer risk using time-resolved diffuse optical spectroscopy". Journal of Biomedical Optics. 15 (6): 060501–060501–3. Bibcode:2010JBO....15f0501T. doi:10.1117/1.3506043. PMID 21198142.
- Quarto, Giovanna; Spinelli, Lorenzo; Pifferi, Antonio; Torricelli, Alessandro; Cubeddu, Rinaldo; Abbate, Francesca; Balestreri, Nicola; Menna, Simona; Cassano, Enrico; Taroni, Paola (18 September 2014). "Estimate of tissue composition in malignant and benign breast lesions by time-domain optical mammography". Biomedical Optics Express. 5 (10): 3684–3698. doi:10.1364/BOE.5.003684. PMC 4206334. PMID 25360382.
- Jiang, Shudong; Pogue, Brian W.; Carpenter, Colin M.; Poplack, Steven P.; Wells, Wendy A.; Kogel, Christine A.; Forero, Jorge A.; Muffly, Lori S.; Schwartz, Gary N.; Paulsen, Keith D.; Kaufman, Peter A. (August 2009). "Evaluation of Breast Tumor Response to Neoadjuvant Chemotherapy with Tomographic Diffuse Optical Spectroscopy: Case Studies of Tumor Region-of-Interest Changes". Radiology. 252 (2): 551–560. doi:10.1148/radiol.2522081202. PMC 2753781. PMID 19508985.
- Cerussi, A.; Hsiang, D.; Shah, N.; Mehta, R.; Durkin, A.; Butler, J.; Tromberg, B. J. (28 February 2007). "Predicting response to breast cancer neoadjuvant chemotherapy using diffuse optical spectroscopy". Proceedings of the National Academy of Sciences. 104 (10): 4014–4019. Bibcode:2007PNAS..104.4014C. doi:10.1073/pnas.0611058104. PMC 1805697. PMID 17360469.
- Martelli, Fabrizio; Del Bianco, Samuele; Ismaelli, Andrea; Zaccanti, Giovanni (2010). Light propagation through biological tissue and other diffusive media : theory, solutions, and software. SPIE. ISBN 9780819476586.
- Yang, Kai; Kwan, Alexander L. C.; Boone, John M. (15 May 2007). "Computer modeling of the spatial resolution properties of a dedicated breast CT system". Medical Physics. 34 (6Part1): 2059–2069. Bibcode:2007MedPh..34.2059Y. doi:10.1118/1.2737263. PMC 2838398. PMID 17654909.
- Dobruch-Sobczak, Katarzyna; Piotrzkowska-Wróblewska, Hanna; Klimoda, Ziemowit; Secomski, Wojciech; Karwat, Piotr; Markiewicz-Grodzicka, Ewa; Kolasińska-Ćwikła, Agnieszka; Roszkowska-Purska, Katarzyna; Litniewski, Jerzy (28 June 2019). "Monitoring the response to neoadjuvant chemotherapy in patients with breast cancer using ultrasound scattering coefficient: A preliminary report". Journal of Ultrasonography. 19 (77): 89–97. doi:10.15557/JoU.2019.0013. PMC 6750328. PMID 31355579. S2CID 198295706.
- Marshall, Eliot (18 February 2010). "Brawling Over Mammography". Science. 327 (5968): 936–938. doi:10.1126/science.327.5968.936. PMID 20167758.
- Grosenick, Dirk; Rinneberg, Herbert; Cubeddu, Rinaldo; Taroni, Paola (11 July 2016). "Review of optical breast imaging and spectroscopy". Journal of Biomedical Optics. 21 (9): 091311. Bibcode:2016JBO....21i1311G. doi:10.1117/1.JBO.21.9.091311. PMID 27403837. S2CID 42000848.
- Kaplan, Stuart S. (December 2001). "Clinical Utility of Bilateral Whole-Breast US in the Evaluation of Women with Dense Breast Tissue". Radiology. 221 (3): 641–649. doi:10.1148/radiol.2213010364. PMID 11719658.
- Hylton, Nola (10 March 2005). "Magnetic Resonance Imaging of the Breast: Opportunities to Improve Breast Cancer Management". Journal of Clinical Oncology. 23 (8): 1678–1684. doi:10.1200/JCO.2005.12.002. PMID 15755976.
- Lord, S.J.; Lei, W.; Craft, P.; Cawson, J.N.; Morris, I.; Walleser, S.; Griffiths, A.; Parker, S.; Houssami, N. (September 2007). "A systematic review of the effectiveness of magnetic resonance imaging (MRI) as an addition to mammography and ultrasound in screening young women at high risk of breast cancer". European Journal of Cancer. 43 (13): 1905–1917. doi:10.1016/j.ejca.2007.06.007. PMID 17681781.
- Bénard, François; Turcotte, Éric (12 May 2005). "Imaging in breast cancer: Single-photon computed tomography and positron-emission tomography". Breast Cancer Research. 7 (4): 153–62. doi:10.1186/bcr1201. PMC 1175073. PMID 15987467.
- Taroni, Paola (2012). "Diffuse optical imaging and spectroscopy of the breast: A brief outline of history and perspectives". Photochem. Photobiol. Sci. 11 (2): 241–250. doi:10.1039/c1pp05230f. PMID 22094324.
- Jacques, Steven L (7 June 2013). "Optical properties of biological tissues: a review". Physics in Medicine and Biology. 58 (11): R37–R61. Bibcode:2013PMB....58R..37J. doi:10.1088/0031-9155/58/11/R37. PMID 23666068.
- Wang, Xin; Pogue, Brian W.; Jiang, Shudong; Song, Xiaomei; Paulsen, Keith D.; Kogel, Christine; Poplack, Steven P.; Wells, Wendy A. (2005). "Approximation of Mie scattering parameters in near-infrared tomography of normal breast tissue in vivo". Journal of Biomedical Optics. 10 (5): 051704. Bibcode:2005JBO....10e1704W. doi:10.1117/1.2098607. PMID 16292956. S2CID 45813277.
- Taroni, Paola; Quarto, Giovanna; Pifferi, Antonio; Abbate, Francesca; Balestreri, Nicola; Menna, Simona; Cassano, Enrico; Cubeddu, Rinaldo; Batra, Surinder K. (1 June 2015). "Breast Tissue Composition and Its Dependence on Demographic Risk Factors for Breast Cancer: Non-Invasive Assessment by Time Domain Diffuse Optical Spectroscopy". PLOS ONE. 10 (6): e0128941. Bibcode:2015PLoSO..1028941T. doi:10.1371/journal.pone.0128941. PMC 4452361. PMID 26029912.
- Provenzano, Paolo P; Inman, David R; Eliceiri, Kevin W; Knittel, Justin G; Yan, Long; Rueden, Curtis T; White, John G; Keely, Patricia J (28 April 2008). "Collagen density promotes mammary tumor initiation and progression". BMC Medicine. 6 (1): 11. doi:10.1186/1741-7015-6-11. PMC 2386807. PMID 18442412.
- Taroni, Paola; Pifferi, Antonio; Torricelli, Alessandro; Comelli, Daniela; Cubeddu, Rinaldo (2003). "In vivo absorption and scattering spectroscopy of biological tissues". Photochemical & Photobiological Sciences. 2 (2): 124–129. doi:10.1039/B209651J. PMID 12664972.
- Durduran, T.; Choe, R.; Culver, J. P.; Zubkov, L.; Holboke, M. J.; Giammarco, J.; Chance, B.; Yodh, A. G. (21 August 2002). "Bulk optical properties of healthy female breast tissue". Physics in Medicine and Biology. 47 (16): 2847–2861. Bibcode:2002PMB....47.2847D. doi:10.1088/0031-9155/47/16/302. PMID 12222850.
- Matcher, Stephen J. (25 October 2016). "Signal Quantification and Localization in Tissue Near-Infrared Spectroscopy". Handbook of Optical Biomedical Diagnostics, Second Edition, Volume 1: Light-Tissue Interaction. pp. 585–687. doi:10.1117/3.2219603.ch9. ISBN 9781628419092.
- Jiang, Huabei; Iftimia, Nicusor V.; Xu, Yong; Eggert, Julia A.; Fajardo, Laurie L.; Klove, Karen L. (February 2002). "Near-Infrared Optical Imaging of the Breast with Model-Based Reconstruction". Academic Radiology. 9 (2): 186–194. doi:10.1016/s1076-6332(03)80169-1. PMID 11918371.
- Xu, Ronald X; Young, Donn C; Mao, Jimmy J; Povoski, Stephen P (18 December 2007). "A prospective pilot clinical trial evaluating the utility of a dynamic near-infrared imaging device for characterizing suspicious breast lesions". Breast Cancer Research. 9 (6): R88. doi:10.1186/bcr1837. PMC 2246191. PMID 18088411. S2CID 3323560.
- Ferocino, Edoardo; Martinenghi, Edoardo; Dalla Mora, Alberto; Pifferi, Antonio; Cubeddu, Rinaldo; Taroni, Paola (23 January 2018). "High throughput detection chain for time domain optical mammography". Biomedical Optics Express. 9 (2): 755–770. doi:10.1364/BOE.9.000755. PMC 5854076. PMID 29552410.
- Enfield, Louise C.; Gibson, Adam P.; Everdell, Nicholas L.; Delpy, David T.; Schweiger, Martin; Arridge, Simon R.; Richardson, Caroline; Keshtgar, Mohammad; Douek, Michael; Hebden, Jeremy C. (18 May 2007). "Three-dimensional time-resolved optical mammography of the uncompressed breast". Applied Optics. 46 (17): 3628–38. Bibcode:2007ApOpt..46.3628E. doi:10.1364/AO.46.003628. PMID 17514325.
- Bevilacqua, Frédéric; Berger, Andrew J.; Cerussi, Albert E.; Jakubowski, Dorota; Tromberg, Bruce J. (1 December 2000). "Broadband absorption spectroscopy in turbid media by combined frequency-domain and steady-state methods". Applied Optics. 39 (34): 6498–6907. Bibcode:2000ApOpt..39.6498B. doi:10.1364/AO.39.006498. PMID 18354663.
- Becker, Wolfgang; Bergmann, Axel; Biscotti, Giovanni Luca; Rueck, Angelika (2004). "Advanced time-correlated single photon counting techniques for spectroscopy and imaging in biomedical systems". In Neev, Joseph; Schaffer, Christopher B; Ostendorf, Andreas (eds.). Commercial and Biomedical Applications of Ultrafast Lasers IV. Vol. 5340. International Society for Optics and Photonics. pp. 104–112. doi:10.1117/12.529143. S2CID 17283884.
- Chance, B.; Cooper, C. E.; Delpy, D. T.; Reynolds, E. O. R.; Tromberg, Bruce J.; Coquoz, Olivier; Fishkin, Joshua B.; Pham, Tuan; Anderson, Eric R.; Butler, John; Cahn, Mitchell; Gross, Jeffrey D.; Venugopalan, Vasan; Pham, David (29 June 1997). "Non–invasive measurements of breast tissue optical properties using frequency–domain photon migration". Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences. 352 (1354): 661–668. Bibcode:1997RSPTB.352..661T. doi:10.1098/rstb.1997.0047. PMC 1691955. PMID 9232853.
- Grosenick, Dirk; Wabnitz, Heidrun; Rinneberg, Herbert H.; Moesta, K. Thomas; Schlag, Peter M. (1 May 1999). "Development of a time-domain optical mammograph and first in vivo applications". Applied Optics. 38 (13): 2927–43. Bibcode:1999ApOpt..38.2927G. doi:10.1364/AO.38.002927. PMID 18319875.
- Moesta, KT; Fantini, S; Jess, H; Totkas, S; Franceschini, MA; Kaschke, M; Schlag, PM (April 1998). "Contrast features of breast cancer in frequency-domain laser scanning mammography". Journal of Biomedical Optics. 3 (2): 129–36. Bibcode:1998JBO.....3..129M. doi:10.1117/1.429869. PMID 23015049.
- Simick, Michelle K.; Jong, Roberta; Wilson, Brian; Lilge, Lothar (2004). "Non-ionizing near-infrared radiation transillumination spectroscopy for breast tissue density and assessment of breast cancer risk". Journal of Biomedical Optics. 9 (4): 794–803. Bibcode:2004JBO.....9..794S. doi:10.1117/1.1758269. PMID 15250768.
- Blackmore, Kristina M.; Knight, Julia A.; Walter, Jane; Lilge, Lothar; Ho, Yuan-Soon (15 January 2015). "The Association between Breast Tissue Optical Content and Mammographic Density in Pre- and Post-Menopausal Women". PLOS ONE. 10 (1): e0115851. Bibcode:2015PLoSO..1015851B. doi:10.1371/journal.pone.0115851. PMC 4295879. PMID 25590139. S2CID 15113061.
- Leff, Daniel Richard; Warren, Oliver J.; Enfield, Louise C.; Gibson, Adam; Athanasiou, Thanos; Patten, Darren K.; Hebden, Jem; Yang, Guang Zhong; Darzi, Ara (28 April 2007). "Diffuse optical imaging of the healthy and diseased breast: A systematic review". Breast Cancer Research and Treatment. 108 (1): 9–22. doi:10.1007/s10549-007-9582-z. PMID 17468951. S2CID 10705543.
- Grosenick, Dirk; Moesta, K Thomas; Möller, Michael; Mucke, Jörg; Wabnitz, Heidrun; Gebauer, Bernd; Stroszczynski, Christian; Wassermann, Bernhard; Schlag, Peter M; Rinneberg, Herbert (7 June 2005). "Time-domain scanning optical mammography: I. Recording and assessment of mammograms of 154 patients". Physics in Medicine and Biology. 50 (11): 2429–2449. Bibcode:2005PMB....50.2429G. doi:10.1088/0031-9155/50/11/001. PMID 15901947.
- Choe, Regine; Konecky, Soren D.; Corlu, Alper; Lee, Kijoon; Durduran, Turgut; Busch, David R.; Pathak, Saurav; Czerniecki, Brian J.; Tchou, Julia; Fraker, Douglas L.; DeMichele, Angela; Chance, Britton; Arridge, Simon R.; Schweiger, Martin; Culver, Joseph P.; Schnall, Mitchell D.; Putt, Mary E.; Rosen, Mark A.; Yodh, Arjun G. (2009). "Differentiation of benign and malignant breast tumors by in-vivo three-dimensional parallel-plate diffuse optical tomography". Journal of Biomedical Optics. 14 (2): 024020. Bibcode:2009JBO....14b4020C. doi:10.1117/1.3103325. PMC 2782703. PMID 19405750.
- Zhu, Quing; Cronin, Edward B.; Currier, Allen A.; Vine, Hugh S.; Huang, Minming; Chen, NanGuang; Xu, Chen (October 2005). "Benign versus Malignant Breast Masses: Optical Differentiation with US-guided Optical Imaging Reconstruction". Radiology. 237 (1): 57–66. doi:10.1148/radiol.2371041236. PMC 1533766. PMID 16183924.
- Wang, Shushu; Zhang, Yi; Yang, Xinhua; Fan, Linjun; Qi, Xiaowei; Chen, Qingqiu; Jiang, Jun (2013). "Shrink pattern of breast cancer after neoadjuvant chemotherapy and its correlation with clinical pathological factors". World Journal of Surgical Oncology. 11 (1): 166. doi:10.1186/1477-7819-11-166. PMC 3728037. PMID 23883300. S2CID 6217814.
- Soliman, H.; Gunasekara, A.; Rycroft, M.; Zubovits, J.; Dent, R.; Spayne, J.; Yaffe, M. J.; Czarnota, G. J. (20 April 2010). "Functional Imaging Using Diffuse Optical Spectroscopy of Neoadjuvant Chemotherapy Response in Women with Locally Advanced Breast Cancer". Clinical Cancer Research. 16 (9): 2605–2614. doi:10.1158/1078-0432.CCR-09-1510. PMID 20406836. S2CID 1275542.
- Roblyer, D.; Ueda, S.; Cerussi, A.; Tanamai, W.; Durkin, A.; Mehta, R.; Hsiang, D.; Butler, J. A.; McLaren, C.; Chen, W.-P.; Tromberg, B. (18 August 2011). "Optical imaging of breast cancer oxyhemoglobin flare correlates with neoadjuvant chemotherapy response one day after starting treatment". Proceedings of the National Academy of Sciences. 108 (35): 14626–14631. Bibcode:2011PNAS..10814626R. doi:10.1073/pnas.1013103108. PMC 3167535. PMID 21852577.