Eberconazole
Eberconazole is an antifungal drug. As a 1% topical cream, it is an effective treatment for dermatophytosis, candidiasis, and pityriasis.[1][2][3]
 | |
| Clinical data | |
|---|---|
| Trade names | Ebernet |
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
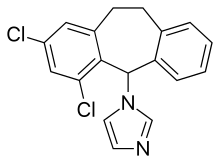
| Formula | C18H14Cl2N2 |
| Molar mass | 329.22 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
It was approved for use in Spain in 2015 and is sold under the trade name Ebernet.[4] It is also approved for use in Panama, Guatemala, Costa Rica, Honduras, and the Dominican Republic.[1]
References
- "Ebernet". NewBridge Pharmaceuticals. Archived from the original on 2016-04-24. Retrieved 2017-01-15.
- del Palacio A, Ortiz FJ, Pérez A, Pazos C, Garau M, Font E (2001). "A double-blind randomized comparative trial: eberconazole 1% cream versus clotrimazole 1% cream twice daily in Candida and dermatophyte skin infections". Mycoses. 44 (5): 173–80. doi:10.1046/j.1439-0507.2001.00632.x. PMID 11486455.
- Repiso Montero T, López S, Rodríguez C, del Rio R, Badell A, Gratacós MR (May 2006). "Eberconazole 1% cream is an effective and safe alternative for dermatophytosis treatment: multicenter, randomized, double-blind, comparative trial with miconazole 2% cream". International Journal of Dermatology. 45 (5): 600–4. doi:10.1111/j.1365-4632.2006.02841.x. PMID 16700802.
- "Eberconazole". Drugs.com.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.