FibroTest
FibroTest, known as FibroSure in the US, is a biomarker test that uses the results of six blood serum tests to generate a score that is correlated with the degree of liver damage in people with a variety of liver diseases. FibroTest has the same prognostic value as a liver biopsy. FibroSure uses quantitative results of five serum biochemical markers, α2-macroglobulin, haptoglobin, apolipoprotein A1, bilirubin, gamma glutamyl transpeptidase (GGT), with a patient’s age and gender to generate a measure of fibrosis and necroinflammatory activity in the liver.
| FibroTest | |
|---|---|
| Purpose | assess degree of liver damage |
FibroTest has been evaluated in relation to liver biopsy (the current reference standard in liver disease assessment) in people with hepatitis C, hepatitis B,[1] alcoholic liver disease,[2] and non-alcoholic fatty liver disease.[3] They are most useful for cirrhosis and less useful for other stages of liver disease.[4]
By 2008 it had been used in over 350,000 patients.[5] In 2006, the French National Authority for Health recommended the use of FibroTest as one of a number of first-line assessment tool for fibrosis with untreated chronic hepatitis C.[6]
Procedure
The FibroTest score is calculated from the results of a six-parameter blood test, combining six serum markers with the age and gender of the patient: Alpha-2-macroglobulin, Haptoglobin, Apolipoprotein A1, Gamma-glutamyl transpeptidase (GGT), Total bilirubin, and Alanine transaminase (ALT). ALT is used in a second assessment called ActiTest that is part of FibroTest.
The equation for calculating the FibroTest score regression coefficient (logistic regression) is:[7]
where B=1 for male and B=0 for female. The score (between 0 and 1) is then
Due to variability of components assays and analyzers, FibroTest assays can only be performed in validated laboratories.[8] FibroTest cannot be used without algorithms that detects false positives and false negatives; the equation alone is not a diagnosis tool.
The laboratory or physician connects to the BioPredictive website[9] for calculation of the test results and prints the results sheet, which is available immediately and is accompanied by an interpretation aid and precautions for use.
Applicability
Over 95% of tests are interpretable and allow a diagnosis of fibrosis and liver activity. In less than 5% of cases, likely false positives or false negatives are highlighted. FibroTest has been validated for chronic hepatitis C,[10] chronic hepatitis B,[5] chronic hepatitis C or B with HIV co-infection,[11] alcoholic liver diseases (steatosis and steatohepatitis),[2] and non-alcoholic steatohepatitis (diabetes, overweight, hypertriglyceridemia, hypercholesterolemia, hypertension).[3]
FibroTest is independent of ethnic origin, sex, genotype, viral load, transaminases or the presence of comorbidities. The test has been validated in those over the age of 65 years,[12] children,[13] people with chronic kidney disease or kidney transplantation, hemophiliacs, patients with chronic inflammatory disease, and the general population.
The tests are not applicable in 1 to 5% of cases. These cases can be detected by laboratory safety algorithms and when detected they are indicated on the results sheet:[5]
- Acute hepatitides, e.g., acute viral hepatitis A, B, C, D, E; drug-induced hepatitis
- Extrahepatic cholestasis, e.g., pancreatic cancer, gallstones
- Severe hemolysis, e.g., some heart valves
- Gilbert's syndrome with high unconjugated hyperbilirubinemia
- Acute inflammatory syndrome (the blood test may be postponed)
Interpretation
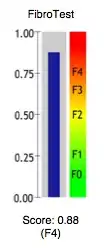
The conversion of FibroTest score into stages according to the three most used histological classifications (METAVIR, Knodell and Ishak) for liver biopsies is:
| FibroTest | METAVIR | Knodell | Ishak |
|---|---|---|---|
| 0.75-1.00 | F4 | F4 | F6 |
| 0.73-0.74 | F3-F4 | F3-F4 | F5 |
| 0.59-0.72 | F3 | F3 | F4 |
| 0.49-0.58 | F2 | F1-F3 | F3 |
| 0.32-0.48 | F1-F2 | F1-F3 | F2-F3 |
| 0.28-0.31 | F1 | F1 | F2 |
| 0.22-0.27 | F0-F1 | F0-F1 | F1 |
| 0.00-0.21 | F0 | F0 | F0 |
Comparison with liver biopsy
Liver biopsy is an imperfect tool; due to sampling errors, biopsy size (5 to 30 mm) and intra- and interobservor variability, it is now agreed that biopsy is an "imperfect Gold Standard " (citation required). Biopsy continues to present inconveniences: 30% of patients complain of pain, up to 3% have been noted to have complications severe enough to require hospitalization[14][15] and a 0.01-0.3% rate of deaths has been reported.[16][17][18] There is a mean discordance of 25% between FibroTest and biopsy. Half of these discordances are attributable to an error of the biopsy, often too small, and the other half to FibroTest.[19] The inventors report that FibroTest has comparable diagnostic and prognostic value as a 25 mm biopsy, while being noninvasive and easily repeatable.[10][2][20]
References
- Houot, M; Ngo, Y; Munteanu, M; Marque, S; Poynard, T (January 2016). "Systematic review with meta-analysis: direct comparisons of biomarkers for the diagnosis of fibrosis in chronic hepatitis C and B." Alimentary Pharmacology & Therapeutics. 43 (1): 16–29. doi:10.1111/apt.13446. PMC 4737301. PMID 26516104.
- Naveau S (2009). "Diagnostic and prognostic values of non-invasive biomarkers of fibrosis in patients with alcoholic liver disease". Clin Gastroenterol Hepatol. 49 (1): 97–105. doi:10.1002/hep.22576. PMID 19053048. S2CID 5782597.
- Ratziu; et al. (2006). "Diagnostic value of biochemical markers (FibroTest-FibroSURE) for the prediction of liver fibrosis in patients with non-alcoholic fatty liver disease". BMC Gastroenterology. 6: 6. doi:10.1186/1471-230X-6-6. PMC 1386692. PMID 16503961.

- Shaheen, AA; Wan, AF; Myers, RP (November 2007). "FibroTest and FibroScan for the prediction of hepatitis C-related fibrosis: a systematic review of diagnostic test accuracy". The American Journal of Gastroenterology. 102 (11): 2589–600. PMID 17850410.
- Halfon P, Munteanu M, Poynard T (2008). "FibroTest-ActiTest as a non-invasive marker of liver fibrosis". Gastroenterol Clin Biol. 32 (6): 22–39. doi:10.1016/S0399-8320(08)73991-5. PMID 18973844.
- Publication of 'Haute Autorité de Santé' (fr)
- "U.S. patent 6,631,330". Archived from the original on 2012-10-11. Retrieved 2011-01-23.
- Imbert-Bismut F; Messous D; Thibault V; Myers RB; Piton A; Thabut D; et al. (2004). "Intra-laboratory analytical variability of biochemical markers of fibrosis (Fibrotest) and activity (Actitest) and references ranges in healthy blood donors". Clin Chem Lab Med. 42 (3): 323–333. doi:10.1515/CCLM.2004.058. PMID 15080567. S2CID 21074850.
- FibroTest website
- Ngo Y, Munteanu M, Messous D, Charlotte F, Imbert-Bismut F, Thabut D, Lebray P, Thibault V, Benhamou Y, Moussalli J, Ratziu V, Poynard T (2006). "A prospective analysis of the prognostic value of biomarkers (FibroTest) in patients with chronic hepatitis C". Clin Chem. 52 (10): 1887–96. doi:10.1373/clinchem.2006.070961. PMID 16931569.
- Cacoub P, Carrat F, Bédossa P, Lambert J, Pénaranda G, Perronne C, Pol S, Halfon P (2008). "Comparison of non-invasive liver fibrosis biomarkers in HIV/HCV co-infected patients: The Fibrovic study - ANRS HC02". J. Hepatol. 48 (5): 765–73. doi:10.1016/j.jhep.2008.01.025. PMID 18314219.
- Thabut; et al. (2006). "Hepatitis C in 6,865 patients 65 yr or older: a severe and neglected curable disease". Am J Gastroenterol. 101 (6): 1260–7. PMID 16771947.
- Hermeziu B; et al. (2009). "Evaluation of FibroTest-ActiTest in children with chronic hepatitis C virus infection". Gastroenterol Clin Biol. 34 (1): 16–22. doi:10.1016/j.gcb.2009.06.007. PMID 19726147.
- Janes, CH & Lindor, KD (1993). "Outcome of patients hospitalized for complications after outpatient liver biopsy". Ann Intern Med. 118 (2): 96–8. doi:10.7326/0003-4819-118-2-199301150-00003. PMID 8416324. S2CID 37740050.
- Pasha T, Gabriel S, Therneau T, Dickson ER, Lindor KD (1998). "Cost-effectiveness of ultrasound-guided liver biopsy". Hepatology. 27 (5): 1220–6. doi:10.1002/hep.510270506. PMID 9581674.
- Strassburg CP, Manns MP (2006). "Approaches to liver biopsy techniques-revisited". Semin Liver Dis. 26 (4): 318–27. doi:10.1055/s-2006-951599. PMID 17051446.
- Gilmore IT; Burroughs A; Murray-Lyon IM; Williams R; Jenkins D; Hopkins A (1995). "Indications, methods, and outcomes of percutaneous liver biopsy in England and Wales: an audit by the British Society of Gastroenterology and the Royal College of Physicians of London". Gut. 36 (3): 437–41. doi:10.1136/gut.36.3.437. PMC 1382461. PMID 7698705.
- Froehlich F; Lamy O; Fried M; Gonvers JJ (1993). "Practice and complications of liver biopsy. Results of a nationwide survey in Switzerland". Dig Dis Sci. 38 (8): 1480–4. doi:10.1007/bf01308607. PMID 8344104. S2CID 21781162.
- Poynard; et al. (2004). "Prospective analysis of discordant results between biochemical markers and biopsy in patients with chronic hepatitis C". Clin Chem. 50 (8): 1344–55. doi:10.1373/clinchem.2004.032227. PMID 15192028.
- Ngo; et al. (2008). Ahmed, Niyaz (ed.). "An accurate definition of the status of inactive hepatitis B virus carrier by a combination of biomarkers (FibroTest-ActiTest) and viral load". PLOS ONE. 3 (7): e2573. Bibcode:2008PLoSO...3.2573N. doi:10.1371/journal.pone.0002573. PMC 2440801. PMID 18596917.
