Gelatin
Gelatin or gelatine (from Latin: gelatus meaning "stiff" or "frozen") is a translucent, colorless, flavorless food ingredient, commonly derived from collagen taken from animal body parts. It is brittle when dry and rubbery when moist. It may also be referred to as hydrolyzed collagen, collagen hydrolysate, gelatine hydrolysate, hydrolyzed gelatine, and collagen peptides after it has undergone hydrolysis. It is commonly used as a gelling agent in food, beverages, medications, drug or vitamin capsules, photographic films, papers, and cosmetics.

Substances containing gelatin or functioning in a similar way are called gelatinous substances. Gelatin is an irreversibly hydrolyzed form of collagen, wherein the hydrolysis reduces protein fibrils into smaller peptides; depending on the physical and chemical methods of denaturation, the molecular weight of the peptides falls within a broad range. Gelatin is present in gelatin desserts, most gummy candy and marshmallows, ice creams, dips, and yogurts.[1] Gelatin for cooking comes as powder, granules, and sheets. Instant types can be added to the food as they are; others must soak in water beforehand.
Characteristics
Properties
Gelatin is a collection of peptides and proteins produced by partial hydrolysis of collagen extracted from the skin, bones, and connective tissues of animals such as domesticated cattle, chicken, pigs, and fish. During hydrolysis, some of the bonds between and within component proteins are broken. Its chemical composition is, in many aspects, closely similar to that of its parent collagen.[2] Photographic and pharmaceutical grades of gelatin generally are sourced from cattle bones and pig skin. Gelatin is classified as a hydrogel.

Gelatin is nearly tasteless and odorless with a colorless or slightly yellow appearance.[3][4] It is transparent and brittle, and it can come as sheets, flakes, or as a powder.[3] Polar solvents like hot water, glycerol, and acetic acid can dissolve gelatin, but it is insoluble in organic solvents like alcohol.[3] Gelatin absorbs 5–10 times its weight in water to form a gel.[3] The gel formed by gelatin can be melted by reheating, and it has an increasing viscosity under stress (thixotropic).[3] The upper melting point of gelatin is below human body temperature, a factor that is important for mouthfeel of foods produced with gelatin.[5] The viscosity of the gelatin-water mixture is greatest when the gelatin concentration is high and the mixture is kept cool at about 4 °C (39 °F). Commercial gelatin will have a gel strength of around 90 to 300 grams Bloom using the Bloom test of gel strength.[6] Gelatin's strength (but not viscosity) declines if it is subjected to temperatures above 100 °C (212 °F), or if it is held at temperatures near 100 °C for an extended period of time.[7][8]
Gelatins have diverse melting points and gelation temperatures, depending on the source. For example, gelatin derived from fish has a lower melting and gelation point than gelatin derived from beef or pork.[9]
Composition
When dry, gelatin consists of 98–99% protein, but it is not a nutritionally complete protein since it is missing tryptophan and is deficient in isoleucine, threonine, and methionine.[10] The amino acid content of hydrolyzed collagen is the same as collagen. Hydrolyzed collagen contains 19 amino acids, predominantly glycine (Gly) 26–34%, proline (Pro) 10–18%, and hydroxyproline (Hyp) 7–15%, which together represent around 50% of the total amino acid content.[11] Glycine is responsible for close packing of the chains. Presence of proline restricts the conformation. This is important for gelation properties of gelatin.[12] Other amino acids that contribute highly include: alanine (Ala) 8–11%; arginine (Arg) 8–9%; aspartic acid (Asp) 6–7%; and glutamic acid (Glu) 10–12%.[11]
Research
Digestibility
A 2005 study in humans found hydrolyzed collagen absorbed as small peptides in the blood.[13]
Effects on skin
Ingestion of hydrolyzed collagen may affect the skin by increasing the density of collagen fibrils and fibroblasts, thereby stimulating collagen production.[14] It has been suggested, based on mouse and in vitro studies, that hydrolyzed collagen peptides have chemotactic properties on fibroblasts[15] or an influence on growth of fibroblasts.[16]
Joint effects
Some clinical studies report that the oral ingestion of hydrolyzed collagen decreases joint pain, those with the most severe symptoms showing the most benefit.[17][18][19]
However, other clinical trials have yielded mixed results. In 2011, the European Food Safety Authority Panel on Dietetic Products, Nutrition and Allergies concluded that "a cause and effect relationship has not been established between the consumption of collagen hydrolysate and maintenance of joints".[20] Four other studies reported benefit with no side effects; however, the studies were not extensive, and all recommended further controlled study.[21][22][23][24] One study found that oral collagen only improved symptoms in a minority of patients and reported nausea as a side effect.[25] Another study reported no improvement in disease activity in patients with rheumatoid arthritis.[26] Another study found that collagen treatment may actually cause an exacerbation of rheumatoid arthritis symptoms.[27]
Safety concerns
Hydrolyzed collagen, like gelatin, is made from animal by-products from the meat industry or sometimes animal carcasses removed and cleared by knackers, including skin, bones, and connective tissue.
In 1997, the U.S. Food and Drug Administration (FDA), with support from the TSE (transmissible spongiform encephalopathy) Advisory Committee, began monitoring the potential risk of transmitting animal diseases, especially bovine spongiform encephalopathy (BSE), commonly known as mad cow disease.[28] An FDA study from that year stated: "...steps such as heat, alkaline treatment, and filtration could be effective in reducing the level of contaminating TSE agents; however, scientific evidence is insufficient at this time to demonstrate that these treatments would effectively remove the BSE infectious agent if present in the source material."[29] On 18 March 2016 the FDA finalized three previously-issued interim final rules designed to further reduce the potential risk of BSE in human food.[30] The final rule clarified that "gelatin is not considered a prohibited cattle material if it is manufactured using the customary industry processes specified."[31]
The Scientific Steering Committee (SSC) of the European Union in 2003 stated that the risk associated with bovine bone gelatin is very low or zero.[32][33]
In 2006, the European Food Safety Authority stated that the SSC opinion was confirmed, that the BSE risk of bone-derived gelatin was small, and that it recommended removal of the 2003 request to exclude the skull, brain, and vertebrae of bovine origin older than 12 months from the material used in gelatin manufacturing.[34]
Production

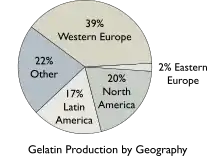
The worldwide demand of gelatin was about 620,000 tonnes (1.4×109 lb) in 2019.[35] On a commercial scale, gelatin is made from by-products of the meat and leather industries. Most gelatin is derived from pork skins, pork and cattle bones, or split cattle hides.[36] Gelatin made from fish by-products avoids some of the religious objections to gelatin consumption.[5] The raw materials are prepared by different curing, acid, and alkali processes that are employed to extract the dried collagen hydrolysate. These processes may take several weeks, and differences in such processes have great effects on the properties of the final gelatin products.
Gelatin also can be prepared at home. Boiling certain cartilaginous cuts of meat or bones results in gelatin being dissolved into the water. Depending on the concentration, the resulting stock (when cooled) will form a jelly or gel naturally. This process is used for aspic.
While many processes exist whereby collagen may be converted to gelatin, they all have several factors in common. The intermolecular and intramolecular bonds that stabilize insoluble collagen must be broken, and also, the hydrogen bonds that stabilize the collagen helix must be broken.[2] The manufacturing processes of gelatin consists of several main stages:
- Pretreatments to make the raw materials ready for the main extraction step and to remove impurities that may have negative effects on physicochemical properties of the final gelatin product.
- Hydrolysis of collagen into gelatin.
- Extraction of gelatin from the hydrolysis mixture, which usually is done with hot water or dilute acid solutions as a multistage process.
- The refining and recovering treatments including filtration, clarification, evaporation, sterilization, drying, rutting, grinding, and sifting to remove the water from the gelatin solution, to blend the gelatin extracted, and to obtain dried, blended, ground final product.
Pretreatments
If the raw material used in the production of the gelatin is derived from bones, dilute acid solutions are used to remove calcium and other salts. Hot water or several solvents may be used to reduce the fat content, which should not exceed 1% before the main extraction step. If the raw material consists of hides and skin; size reduction, washing, removal of hair from hides, and degreasing are necessary to prepare the hides and skins for the hydrolysis step.
Hydrolysis
After preparation of the raw material, i.e., removing some of the impurities such as fat and salts, partially purified collagen is converted into gelatin through hydrolysis. Collagen hydrolysis is performed by one of three different methods: acid-, alkali-, and enzymatic hydrolysis. Acid treatment is especially suitable for less fully cross-linked materials such as pig skin collagen and normally requires 10 to 48 hours. Alkali treatment is suitable for more complex collagen such as that found in bovine hides and requires more time, normally several weeks. The purpose of the alkali treatment is to destroy certain chemical crosslinks still present in collagen. Within the gelatin industry, the gelatin obtained from acid-treated raw material has been called type-A gelatin and the gelatin obtained from alkali-treated raw material is referred to as type-B gelatin.[37]
Advances are occurring to optimize the yield of gelatin using enzymatic hydrolysis of collagen. The treatment time is shorter than that required for alkali treatment, and results in almost complete conversion to the pure product. The physical properties of the final gelatin product are considered better.[38]
Extraction
Extraction is performed with either water or acid solutions at appropriate temperatures. All industrial processes are based on neutral or acid pH values because although alkali treatments speed up conversion, they also promote degradation processes. Acidic extraction conditions are extensively used in the industry, but the degree of acid varies with different processes. This extraction step is a multistage process, and the extraction temperature usually is increased in later extraction steps, which ensures minimum thermal degradation of the extracted gelatin.
Recovery
This process includes several steps such as filtration, evaporation, drying, grinding, and sifting. These operations are concentration-dependent and also dependent on the particular gelatin used. Gelatin degradation should be avoided and minimized, so the lowest temperature possible is used for the recovery process. Most recoveries are rapid, with all of the processes being done in several stages to avoid extensive deterioration of the peptide structure. A deteriorated peptide structure would result in a low gel strength, which is not generally desired.
Uses
Early history of food applications
The 10th-century Kitab al-Tabikh includes a recipe for a fish aspic, made by boiling fish heads.[39]
A recipe for jelled meat broth is found in Le Viandier, written in or around 1375.[40]
In 15th century Britain, cattle hooves were boiled to produce a gel.[41] By the late 17th century, the French inventor Denis Papin had discovered another method of gelatin extraction via boiling of bones.[42] An English patent for gelatin production was granted in 1754.[41] In 1812, the chemist Jean-Pierre-Joseph d'Arcet (fr) further experimented with the use of hydrochloric acid to extract gelatin from bones, and later with steam extraction, which was much more efficient. The French government viewed gelatin as a potential source of cheap, accessible protein for the poor, particularly in Paris.[43] Food applications in France and the United States during 19th century appear to have established the versatility of gelatin, including the origin of its popularity in the US as Jell-O.[44] From the mid 1800s, Charles and Rose Knox of New York manufactured and marketed gelatin powder, diversifying the appeal and applications of gelatin.[45]
Culinary uses

Probably best known as a gelling agent in cooking, different types and grades of gelatin are used in a wide range of food and nonfood products. Common examples of foods that contain gelatin are gelatin desserts, trifles, aspic, marshmallows, candy corn, and confections such as Peeps, gummy bears, fruit snacks, and jelly babies.[46] Gelatin may be used as a stabilizer, thickener, or texturizer in foods such as yogurt, cream cheese, and margarine; it is used, as well, in fat-reduced foods to simulate the mouthfeel of fat and to create volume. It also is used in the production of several types of Chinese soup dumplings, specifically Shanghainese soup dumplings, or xiaolongbao, as well as Shengjian mantou, a type of fried and steamed dumpling. The fillings of both are made by combining ground pork with gelatin cubes, and in the process of cooking, the gelatin melts, creating a soupy interior with a characteristic gelatinous stickiness.
Gelatin is used for the clarification of juices, such as apple juice, and of vinegar.[47]
Isinglass is obtained from the swim bladders of fish. It is used as a fining agent for wine and beer.[48] Besides hartshorn jelly, from deer antlers (hence the name "hartshorn"), isinglass was one of the oldest sources of gelatin.
Cosmetics
In cosmetics, hydrolyzed collagen may be found in topical creams, acting as a product texture conditioner, and moisturizer. Collagen implants or dermal fillers are also used to address the appearance of wrinkles, contour deficiencies, and acne scars, among others. The U.S. Food and Drug Administration has approved its use, and identifies cow (bovine) and human cells as the sources of these fillers. According to the FDA, the desired effects can last for 3–4 months, which is relatively the most short-lived compared to other materials used for the same purpose.[49]
Other technical uses

- Certain professional and theatrical lighting equipment use color gels to change the beam color. Historically, these were made with gelatin, hence the term, color gel.
- Originally, gelatin constituted the shells of all drug and vitamin capsules to make them easier to swallow. Now, a vegetarian-acceptable alternative to gelatin, hypromellose, is also used, and is less expensive than gelatin to produce.
- Some animal glues such as hide glue may be unrefined gelatin.
- It is used to hold silver halide crystals in an emulsion in virtually all photographic films and photographic papers. Despite significant effort, no suitable substitutes with the stability and low cost of gelatin have been found.
- Used as a carrier, coating, or separating agent for other substances, for example, it makes β-carotene water-soluble, thus imparting a yellow color to any soft drinks containing β-carotene.
- Ballistic gelatin is used to test and measure the performance of bullets shot from firearms.
- Gelatin is used as a binder in match heads[50] and sandpaper.[51]
- Cosmetics may contain a non-gelling variant of gelatin under the name hydrolyzed collagen (hydrolysate).
- Gelatin was first used as an external surface sizing for paper in 1337 and continued as a dominant sizing agent of all European papers through the mid-nineteenth century.[52] In modern times, it is mostly found in watercolor paper, and occasionally in glossy printing papers, artistic papers, and playing cards. It maintains the wrinkles in crêpe paper.
- Biotechnology: Gelatin is also used in synthesizing hydrogels for tissue engineering applications.[53] Gelatin is also used as a saturating agent in immunoassays, and as a coat.[54] Gelatin degradation assay allows visualizing and quantifying invasion at the subcellular level instead of analyzing the invasive behavior of whole cells, for the study of cellular protrusions called invadopodia and podosomes, which are protrusive structures in cancer cells and play an important role in cell attachment and remodeling of the extracellular matrix (ECM).[55]
Religious considerations
The consumption of gelatin from particular animals may be forbidden by religious rules or cultural taboos.
Islamic halal and Jewish kosher customs generally require gelatin from sources other than pigs, such as cattle that have been slaughtered according to religious regulations (halal or kosher), or fish (that Jews and Muslims are allowed to consume).[56]
On the other hand, some Islamic jurists have argued that the chemical treatment "purifies" the gelatin enough to always be halal, an argument most common in the field of medicine.[56]
It has similarly been argued that gelatin in medicine is permissible in Judaism, as it is not used as food.[57] According to The Jewish Dietary Laws, the book of kosher guidelines published by the Rabbinical Assembly, the organization of Conservative Jewish rabbis, all gelatin is kosher and pareve because the chemical transformation undergone in the manufacturing process renders it a different physical and chemical substance.[58]
Sikh, Hindu, and Jain customs may require gelatin alternatives from sources other than animals, as many Hindus, most Jains and some Sikhs are vegetarian.[59]
See also
- Agar
- Carrageenan
- Konjac
- Pectin
References
- Kodjo Boady Djagnya; Zhang Wang; Shiying Xu (2010). "Gelatin: A Valuable Protein for Food and Pharmaceutical Industries: Review". Critical Reviews in Food Science and Nutrition. 41 (6): 481–92. doi:10.1080/20014091091904. PMID 11592686. S2CID 37668312.
- Ward, A.G.; Courts, A. (1977). The Science and Technology of Gelatin. New York: Academic Press. ISBN 978-0-12-735050-9.
- Budavari, S. (1996). Merck Index, (12th ed.) Whitehouse Station, NJ: Merck.
- Food and Nutrition Board, National Academy of Sciences. (1996). Food Chemicals Codex 4th Ed. Washington, DC: National Academy Press.
- Francis, Frederick J., ed. (2000). "Gelatin". Encyclopedia of Food Science and Technology (2nd ed.). John Wiley & Sons. pp. 1183–88. ISBN 978-0471192558. Archived from the original on 29 August 2005.
- Igoe, R.S. (1983). Dictionary of Food Ingredients. New York: Van Nostrand Reinhold.
- 6 Unexpected Factors That Can Ruin Your Gelatin Desserts | Serious Eats
- The Science of Gelatin – FineCooking
- "National Organic Standards Board Technical Advisory Panel Review: Gelatin processing" (PDF). omri.org. Archived from the original (PDF) on 27 September 2007.
- Potter, N.N. and J.H. Hotchkiss. (1998). Food Science (5th ed.) Gaithersburg, MD: Aspen.
- Poppe, J. (1997). Gelatin, in A. Imeson (ed.) Thickening and Gelling Agents for Food (2nd ed.): 144–68. London: Blackie Academic and Professional.
- "Gelatin Handbook" (PDF). Archived from the original (PDF) on 16 May 2017. Retrieved 27 September 2017.
- Iwai, K.; Hasegawa, T.; Taguchi, Y.; Morimatsu, F.; Sato, K.; Nakamura, Y.; Higashi, A.; Kido, Y.; Nakabo, Y.; Ohtsuki, K. (2005). "Identification of food-derived collagen peptides in human blood after oral ingestion of gelatine hydrolysates". Journal of Agricultural and Food Chemistry. 53 (16): 6531–36. doi:10.1021/jf050206p. PMID 16076145.
- Matsuda, N.; Koyama, Y.; Hosaka, Y.; Ueda, H.; Watanabe, T.; Araya, T.; Irie, S.; Takehana, K (2006). "Effects of ingestion of collagen peptide on collagen fibrils and glycosaminoglycans in the dermis". Journal of Nutritional Science and Vitaminology. 52 (3): 211–15. doi:10.3177/jnsv.52.211. PMID 16967766.
- Postlethwaite, A. E.; Seyer, J. M.; Kang, A. H. (1978). "Chemotactic attraction of human fibroblasts to type I, II, and III collagens and collagen-derived peptides". Proceedings of the National Academy of Sciences of the United States of America. 75 (2): 871–75. Bibcode:1978PNAS...75..871P. doi:10.1073/pnas.75.2.871. PMC 411359. PMID 204938.
- Shigemura, Y.; K Iwai; F Morimatsu; T Iwamoto; T Mori; C Oda; T Taira; EY Park; Y Nakamura; K Sato (2009). "Effect of prolyl-hydroxyproline (Pro-Hyp), a food-derived collagen peptide in human blood, on growth of fibroblasts from mouse skin". Journal of Agricultural and Food Chemistry. 57 (2): 444–49. doi:10.1021/jf802785h. PMID 19128041.
- Moskowitz, R. (2000). "Role of collagen hydrolysate in bone and joint disease". Seminars in Arthritis and Rheumatism. 30 (2): 87–99. doi:10.1053/sarh.2000.9622. PMID 11071580.
- Ruiz-Benito, P.; Camacho-Zambrano, M.M.; Carrillo-Arcentales, J.N.; Mestanza-Peralta, M.A.; Vallejo-Flores, C.A.; Vargas-Lopez, S.V.; Villacis-Tamayo, R.A.; Zurita-Gavilanes, L.A. (2009). "A randomized controlled trial on the efficacy and safety of a food ingredient, collagen hydrolysate, for improving joint comfort". International Journal of Food Sciences and Nutrition. 12: 1–15. doi:10.1080/09637480802498820. PMID 19212858. S2CID 21412854.
- Zdzieblik D, Oesser S, Gollhofer A, König D (June 2017). "Improvement of activity-related knee joint discomfort following supplementation of specific collagen peptides". Appl Physiol Nutr Metab. 42 (6): 588–95. doi:10.1139/apnm-2016-0390. PMID 28177710.
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA) (2011). "Scientific Opinion on the substantiation of a health claim related to collagen hydrolysate and maintenance of joints pursuant to Article 13(5) of Regulation (EC) No 1924/2006". EFSA Journal. 9 (7). doi:10.2903/j.efsa.2011.2291.
- Barnett ML, Kremer JM, St Clair EW, Clegg DO, Furst D, Weisman M, Fletcher MJ, Chasan-Taber S, Finger E, Morales A, Le CH, Trentham DE (February 1998). "Treatment of rheumatoid arthritis with oral type II collagen. Results of a multicenter, double-blind, placebo-controlled trial". Arthritis Rheum. 41 (2): 290–97. doi:10.1002/1529-0131(199802)41:2<290::AID-ART13>3.0.CO;2-R. PMID 9485087.
- Ausar SF, Beltramo DM, Castagna LF, Quintana S, Silvera E, Kalayan G, Revigliono M, Landa CA, Bianco ID (May 2001). "Treatment of rheumatoid arthritis by oral administration of bovine tracheal type II collagen". Rheumatol. Int. 20 (4): 138–44. doi:10.1007/s002960100099. PMID 11411957. S2CID 44609239.
- Trentham DE, Dynesius-Trentham RA, Orav EJ, Combitchi D, Lorenzo C, Sewell KL, Hafler DA, Weiner HL (September 1993). "Effects of oral administration of type II collagen on rheumatoid arthritis". Science. 261 (5129): 1727–30. Bibcode:1993Sci...261.1727T. doi:10.1126/science.8378772. PMID 8378772.
- Bagchi D, Misner B, Bagchi M, Kothari SC, Downs BW, Fafard RD, Preuss HG (2002). "Effects of orally administered undenatured type II collagen against arthritic inflammatory diseases: a mechanistic exploration". Int J Clin Pharmacol Res. 22 (3–4): 101–10. PMID 12837047.
- Sieper J, Kary S, Sörensen H, Alten R, Eggens U, Hüge W, Hiepe F, Kühne A, Listing J, Ulbrich N, Braun J, Zink A, Mitchison NA (January 1996). "Oral type II collagen treatment in early rheumatoid arthritis. A double-blind, placebo-controlled, randomized trial". Arthritis Rheum. 39 (1): 41–51. doi:10.1002/art.1780390106. PMID 8546737.
- McKown KM, Carbone LD, Kaplan SB, Aelion JA, Lohr KM, Cremer MA, Bustillo J, Gonzalez M, Kaeley G, Steere EL, Somes GW, Myers LK, Seyer JM, Kang AH, Postlethwaite AE (June 1999). "Lack of efficacy of oral bovine type II collagen added to existing therapy in rheumatoid arthritis". Arthritis Rheum. 42 (6): 1204–08. doi:10.1002/1529-0131(199906)42:6<1204::AID-ANR17>3.0.CO;2-U. PMID 10366113.
- Cazzola M, Antivalle M, Sarzi-Puttini P, Dell'Acqua D, Panni B, Caruso I (2000). "Oral type II collagen in the treatment of rheumatoid arthritis. A six-month double blind placebo-controlled study". Clin. Exp. Rheumatol. 18 (5): 571–77. PMID 11072596.
- "Transmissible Spongiform Encephalopathies Advisory Committee (CJDSAC) Meeting Start Date – 23-APR-97" (PDF). Food and Drug Administration. Archived (PDF) from the original on 4 April 2017.
- U.S. Food and Drug Administration. "The Sourcing and Processing of Gelatin to Reduce the Potential Risk Posed by Bovine Spongiform Encephalopathy (BSE) in FDA-Regulated Products for Human Use". Food and Drug Administration. Archived from the original on 21 January 2017.
- Food and Drug Administration (18 March 2016). "Federal Register :: Use of Materials Derived From Cattle in Human Food and Cosmetics". Federal Register, The Daily Journal of the United States Government. Archived from the original on 3 June 2017. Retrieved 24 May 2017.
- U.S. Food and Drug Administration (17 March 2016). "FDA Announces Final Rule on Bovine Spongiform Encephalopathy". Food and Drug Administration. Archived from the original on 30 April 2017. Retrieved 24 May 2017.
Finally, the rule provides a definition of gelatin and clarifies that gelatin is not considered a prohibited cattle material if it is manufactured using the customary industry processes specified. Gelatin was never considered a prohibited cattle material, but FDA had never specifically defined gelatin in past IFRs.
- Scientific Steering Committee, European Union (6–7 March 2003). "Updated Opinion On The Safety With Regard To TSE Risks Of Gelatine Derived From Ruminant Bones or Hides" (PDF). Archived from the original (PDF) on 26 October 2012.
- Gelatine Manufacturers of Europe (GME) (June 2003). "The Removal and Inactivation of Potential TSE Infectivity by the Different Gelatin Manufacturing Processes" (PDF). Food and Drug Administration. Archived (PDF) from the original on 14 January 2012.
- Scientific Panel on Biological Hazards of the European Food Safety Authority (EFSA) (2006). "Quantitative assessment of the human BSE risk posed by gelatine with respect to residual BSE risk". EFSA Journal. 312: 1–29. doi:10.2903/j.efsa.2006.312.
- "Gelatin Market Size, Analysis | Industry Trends Report, 2020-2027". www.grandviewresearch.com. Retrieved 17 October 2020.
- "Natural Health Products Ingredients Database: Hydrolyzed Collagen". Government of Canada, Health Canada, Health Products and Food Branch, Natural Health Products Directorate. 12 June 2013. Archived from the original on 12 May 2016. Retrieved 9 May 2016.
- "Type A & B Process Definition". Vyse Gelatin Company. 26 October 2009. Archived from the original on 1 March 2015. Retrieved 16 July 2014.
- Ahmad, Tanbir; Ismail, Amin; Ahmad, Siti Aqlima; Khalil, Khalilah A.; Kumar, Yogesh; Adeyemi, Kazeem D.; Sazili, Awis Q. (February 2017). "Recent advances on the role of process variables affecting gelatin yield and characteristics with special reference to enzymatic extraction: A review". Food Hydrocolloids. 63: 85–96. doi:10.1016/j.foodhyd.2016.08.007.
- Nasrallah, Nawal (2007). Annals of the Caliphs' Kitchens. Brill.
- Scully, Terence (1 January 1988). The viandier of Taillevent: an edition of all extant manuscripts. Ottawa, Ontario: University of Ottawa Press. p. 270. ISBN 978-0-7766-0174-8.
- "Gelatin". Encyclopedia.com. 2016. Archived from the original on 17 September 2016. Retrieved 9 September 2016.
- Viel, Claude; Fournier, Josette (2006). "Histoire des procédés d'extraction de la gélatine et débats des commissions académiques (XIXe siècle)" [History of gelatin extraction processes and debates of academic commissions]. Revue d'Histoire de la Pharmacie (in French). 54 (349): 7–28. doi:10.3406/pharm.2006.5939. PMID 17152838. Retrieved 2 January 2020.
- Davis, Jennifer J. (2013). Defining Culinary Authority: The Transformation of Cooking in France, 1650–1830. Louisiana State University Press.
- Wyman, Carolyn (2001). Jell-o: A Biography: the History And Mystery of America's Most Famous Dessert. Diane Publishing Company. ISBN 978-0756788544.
- "Gelatin: background". Encyclopedia.com. 2016. Archived from the original on 17 September 2016. Retrieved 9 September 2016.
- Nene, Chhaya (9 March 2018). "Six Popular Foods You Didn't Know Had Gelatin". Medium. Retrieved 13 August 2020.
- Organic Materials Review Institute for the USDA National Organic Program. (2002). "Gelatin: Processing." National Organic Standards Board Technical Advisory Panel Review. https://www.ams.usda.gov/sites/default/files/media/Gelatin%20Fish%20TR%20Review.pdf
- "National Organic Standards Board Technical Advisory Panel Review: Gelatin processing" (PDF). omri.org. Archived from the original (PDF) on 27 September 2007.
- Health, Center for Devices and Radiological (13 June 2019). "Dermal Fillers Approved by the Center for Devices and Radiological Health". FDA.
- Finch, C. A.; Ramachandran, Srinivasa (1983). Matchmaking, science, technology, and manufacture. Ellis Horwood. p. 141. ISBN 978-0853123156.
- Packham, D. E. (2006). Handbook of Adhesion. John Wiley & Sons. p. 48. ISBN 978-0470014219.
- Thurn, Jim. "History, Chemistry, and Long Term Effects of Alum-Rosin Size in Paper". ischool.utexas.edu. Archived from the original on 25 April 2012.
- Rizwan, Muhammad; Peh, Gary S. L.; Ang, Heng-Pei; Lwin, Nyein Chan; Adnan, Khadijah; Mehta, Jodhbir S.; Tan, Wui Siew; Yim, Evelyn K. F. (1 March 2017). "Sequentially-crosslinked bioactive hydrogels as nano-patterned substrates with customizable stiffness and degradation for corneal tissue engineering applications". Biomaterials. 120: 139–54. doi:10.1016/j.biomaterials.2016.12.026. ISSN 0142-9612. PMID 28061402.
- ISOMURA Mitsuo, UENO Masayoshi, SHIMADA Kazuya, ASHIHARA Yoshihiro (8 July 1994). "Magnetic Particles with Gelatin and Immunoassay using the same". Europe PMC. Retrieved 18 June 2021.
{{cite journal}}: CS1 maint: uses authors parameter (link) - Díaz, Begoña (20 December 2013). "Invadopodia Detection and Gelatin Degradation Assay". Bio-Protocol. 3 (24). doi:10.21769/bioprotoc.997. ISSN 2331-8325. PMC 6233998. PMID 30443559.
- Gezairy HA (17 July 2001). "(Form letter EDB.7/3 P6/61/3)" (PDF). World Health Organization, Regional Office for the Eastern Mediterranean. Retrieved 12 May 2009.
- Smith, MJ (November 2015). "Promoting Vaccine Confidence". Infectious Disease Clinics of North America (Review). 29 (4): 759–69. doi:10.1016/j.idc.2015.07.004. PMID 26337737.
- Samuel H. Dresner; Seymour Siegel; David M. Pollock (1982). The Jewish Dietary Laws. The Rabbinical Assembly. p. 97-98. ISBN 978-0-8381-2105-4.
- Schmidt, Arno; Fieldhouse, Paul (2007). The World Religions Cookbook. Greenwood Publishing Group. p. 99. ISBN 978-0-313-33504-4.
External links
![]() Media related to Gelatin at Wikimedia Commons
Media related to Gelatin at Wikimedia Commons