Glucagon rescue
Glucagon rescue is the emergency injection of glucagon in case of severe diabetic hypoglycemia. It is needed during seizures and/or unconsciousness by an insulin user who is unable at that point to help themselves. Glucagon will facilitate the release of stored glucose back into the bloodstream, raising the blood glucose level.
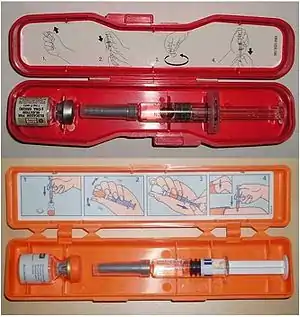
Rescue has been simplified by the development of the glucagon hypoglycemia rescue kit, consisting of:
- biosynthetic human glucagon, in a freeze dried form within a vial,
- a sturdy syringe, pre-filled with a sterile diluting solution, and
- a conspicuous red or orange colored plastic storage box, which includes instructions.
At the first signs of hypoglycemia, an insulin user should treat it immediately by consuming carbohydrate to restore blood glucose to safe levels (thereby preventing progression to severe hypoglycemia). However, not all insulin users can feel and recognize the early signs, particularly when sleeping. This can quickly lead to an emergency resulting in unconsciousness, inability to swallow, seizures, and in extreme cases death. In the past, treatment consisted of intravenous delivery of dextrose (glucose) usually in a hospital emergency department; however, the delay in treatment due to emergency response and transport to a medical facility is life-threatening.
The glucagon rescue kit facilitates rapid rescue by a simple injection, which does not require medical expertise, and can be done quickly and easily outside of a medical facility.[1][2][3]
Glucagon rescue kits
Glucagon rescue kits are manufactured by Novo Nordisk and Eli Lilly and Company. Novo Nordisk manufactures the GlucaGen HypoKit and Eli Lilly and Company manufactures the Glucagon emergency kit.
Potential issues with glucagon administration
 Unrolled, the front |
 Unrolled, the back |
Glucagon must be reconstituted using a multi-step process that requires the administrator to use a prepared syringe containing an inactive compound and a sealed dosage bottle of glucagon. After the contents of the syringe are injected into the glucagon bottle, the administrator must determine if the reconstitution appears appropriate: clear with a water-like consistency. This is a subjective assessment. The solution must be drawn back into the syringe, the injection site cleaned with alcohol, and then injected with sufficient force into fatty tissue. Individuals who have not performed this procedure before are at a disadvantage increased by the potential for severe hypoglycemia [4]
At the present time, there are no ready-made formulations of glucagon; within hours of reconstitution, the molecule will degrade into amyloid fibrils.[5] Combined with the anxiety of performing a rescue is expense, bulkiness, and concerns over dosing correctly;[6] fortunately, there is no biochemical way to overdose on intramuscularly injected glucagon.[4]

Politics of glucagon rescue
Some U.S. union teacher contracts stipulate they shall not be allowed to deliver glucagon or even be trained in administration of glucagon.[7] [8] [9]
Glucagon 'mini-dose' instruction
The purpose of the off-label 'mini-dose' is to avoid an emergency condition that may require glucagon rescue. This might be needed in cases such as when a diabetic child is injected with insulin before breakfast, eats, and then vomits and cannot eat again: with the injected insulin working its way into the bloodstream and no carbohydrate to balance, there may soon be a hypoglycemic emergency.
Medical studies have shown that the mini-dose rescue is tolerated well and effective.[10]
References
- User information for ‘GlucaGen HypoKit’ from Novo Nordisk
- User information for ‘Glucagon Emergency Kit’ from Eli Lilly and Company
- ‘GlucaGen Hypo Kit’ elearning course Archived 13 February 2013 at archive.today
- "Glucagon for Injection, rDNA formulation" (PDF). Lilly. Retrieved 23 February 2014.
- "Stable liquid glucagon formulations for rescue treatment and bi-hormonal closed-loop pancreas." Curr Diab Rep. 2012 Dec;12(6):705-10. doi:10.1007/s11892-012-0320-5
- Muchnick, Jeanne (5 November 2013). "A User-Friendly Glucagon Kit?". InsulinNation. Retrieved 23 February 2014.
- Legal Rights of Students with Diabetes – American Diabetes Association
- Diabetes Care in Daycare: State Laws, Regulations, and Policies Related to Insulin and Glucagon Administration and Other Diabetes Care – American Diabetes Association
- "Know Your Rights". American Diabetes Association. Retrieved 26 May 2016.
- Mini-Dose Glucagon Rescue for Hypoglycemia in Children With Type 1 Diabetes – Haymond and Schreiner. Diabetes Care 24(4):643–45.
Further reading
- Kedia, Nitil (2011). "Treatment of severe diabetic hypoglycemia with glucagon: an underutilized therapeutic approach". Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy. 4: 337–46. doi:10.2147/DMSO.S20633. PMC 3180523. PMID 21969805.
- Pearson, T. (2008). "Glucagon as a Treatment of Severe Hypoglycemia: Safe and Efficacious but Underutilized". The Diabetes Educator. 34 (1): 128–134. doi:10.1177/0145721707312400. PMID 18267999. S2CID 31043845.
- Frier BM. Hypoglycemia in type 2 diabetes. Diabetic Hypoglycemia Jun 2008;2:2-7.
- Harrism, G.; Diment, A.; Sulway, M.; Wilkinson, M. (2001). "Glucagon administration – underevaluated and undertaught". Practical Diabetes International. 18 (1): 22–25. doi:10.1002/pdi.138.
- Namba, Mitsuyoshi; Hanafusa, Toshiaki; Kono, Norio; Tarui, Seiichiro; The GL-G Hypoglycemia Study Group (1993). "Clinical evaluation of biosynthetic glucagon treatment for recovery from hypoglycemia developed in diabetic patients". Diabetes Research and Clinical Practice. 19 (2): 133–138. doi:10.1016/0168-8227(93)90106-F. PMID 8472628.
- Vukmir, Rade B; Paris, Paul M; Yealy, Donald M (1991). "Glucagon: Prehospital therapy for hypoglycemia" (PDF). Annals of Emergency Medicine. 20 (4): 375–379. doi:10.1016/S0196-0644(05)81658-0. PMID 2003665. Archived from the original (PDF) on 3 September 2013.
- Carstens, S; Sprehn, M (April–December 1998). "Prehospital treatment of severe hypoglycaemia: a comparison of intramuscular glucagon and intravenous glucose". Prehospital and Disaster Medicine. 13 (2–4): 44–50. doi:10.1017/s1049023x00030132. PMID 10346406. S2CID 30087320.