Gluconic acid
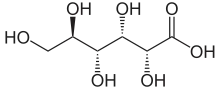
Gluconic acid is an organic compound with molecular formula C6H12O7 and condensed structural formula HOCH2(CHOH)4COOH. It is one of the 16 stereoisomers of 2,3,4,5,6-pentahydroxyhexanoic acid.
 | |
 | |
| Names | |
|---|---|
| IUPAC name
d-Gluconic acid | |
| Systematic IUPAC name
(2R,3S,4R,5R)-2,3,4,5,6-Pentahydroxyhexanoic acid | |
| Other names
Dextronic acid | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.007.639 |
| EC Number |
|
| E number | E574 (acidity regulators, ...) |
PubChem CID |
|
| UNII |
|
CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C6H12O7 |
| Molar mass | 196.155 g·mol−1 |
| Appearance | Colorless crystals |
| Melting point | 131 °C (268 °F; 404 K) |
Solubility in water |
316 g/L[1] |
| Acidity (pKa) | 3.86[2] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
In aqueous solution at neutral pH, gluconic acid forms the gluconate ion. The salts of gluconic acid are known as "gluconates". Gluconic acid, gluconate salts, and gluconate esters occur widely in nature because such species arise from the oxidation of glucose. Some drugs are injected in the form of gluconates.
Chemical structure
The chemical structure of gluconic acid consists of a six-carbon chain, with five hydroxyl groups positioned in the same way as in the open-chained form of glucose, terminating in a carboxylic acid group. In aqueous solution, gluconic acid exists in equilibrium with the cyclic ester glucono delta-lactone.
Production
Gluconic acid preparation was first reported by Hlasiwetz and Habermann in 1870[3] and involved the chemical oxidation of glucose. In 1880, Boutroux prepared and isolated gluconic acid using the glucose fermentation.[4]
Contemporary methods for the gluconic acid production utilize variations of glucose (or other carbohydrate-containing substrate) oxidation using fermentation[5][6] or noble metal catalysis.[7][8]
Occurrence and uses
Gluconic acid occurs naturally in fruit, honey, and wine. As a food additive (E574[9]), it is now known as an acidity regulator.
The gluconate anion chelates Ca2+, Fe2+, K+, Al3+, and other metals, including lanthanides and actinides. It is also used in cleaning products, where it dissolves mineral deposits, especially in alkaline solution.
Zinc gluconate injections are used to neuter male dogs.[10]
Gluconate is also used in building and construction as a concrete admixture (retarder) to slow down the cement hydration reactions, and to delay the cement setting time. It allows for a longer time to lay the concrete, or to spread the cement hydration heat over a longer period of time to avoid too high a temperature and the resulting cracking.[11][12] Retarders are mixed in to concrete when the weather temperature is high or to cast large and thick concrete slabs in successive and sufficiently well-mixed layers.
Gluconic acid aqueous solution finds application as a medium for organic synthesis.[13]
Medicine
In medicine, gluconate is used most commonly as a biologically neutral carrier of Zn2+, Ca2+, Cu2+, Fe2+, and K+ to treat electrolyte imbalance.[14]
Calcium gluconate, in the form of a gel, is used to treat burns from hydrofluoric acid;[15][16] calcium gluconate injections may be used for more severe cases to avoid necrosis of deep tissues, as well as to treat hypocalcemia in hospitalized patients. Gluconate is also an electrolyte present in certain solutions, such as "plasmalyte a", used for intravenous fluid resuscitation.[17] Quinine gluconate is a salt of gluconic acid and quinine, which is used for intramuscular injection in the treatment of malaria.
Ferrous gluconate injections have been proposed in the past to treat anemia.[18]
See also
- Uronic acid
- Glucuronic acid
- Isosaccharinic acid (ISA)
References
- "D-Gluconic acid". American Chemical Society.
- Bjerrum, J., et al. Stability Constants, Chemical Society, London, 1958.
- Hlasiwetz, H.; Habermann, J. (1870). "Zur Kenntniss einiger Zuckerarten. (Glucose, Rohrzucker, Levulose, Sorbin, Phloroglucin.)". Berichte der Deutschen Chemischen Gesellschaft. 3 (1): 486–495. doi:10.1002/cber.187000301162. ISSN 1099-0682.
- Boutroux, L. (1880). "Sur une fermentation nouvelle du glucose". Comptes Rendus de l'Académie des Sciences. 91: 236–238.
- Singh, Om V.; Kumar, Raj (2007). "Biotechnological production of gluconic acid: future implications". Applied Microbiology and Biotechnology. 75 (4): 713–722. doi:10.1007/s00253-007-0851-x. ISSN 1432-0614. PMID 17525864. S2CID 7700011.
- Pal, Parimal; Kumar, Ramesh; Banerjee, Subhamay (2016). "Manufacture of gluconic acid: A review towards process intensification for green production". Chemical Engineering and Processing: Process Intensification. 104: 160–171. doi:10.1016/j.cep.2016.03.009. ISSN 0255-2701.
- Yan, Wenjuan; Zhang, Dongpei; Sun, Yu; Zhou, Ziqi; Du, Yihang; Du, Yiyao; Li, Yushan; Liu, Mengyuan; Zhang, Yuming; Shen, Jian; Jin, Xin (2020). "Structural sensitivity of heterogeneous catalysts for sustainable chemical synthesis of gluconic acid from glucose". Chinese Journal of Catalysis. 41 (9): 1320–1336. doi:10.1016/S1872-2067(20)63590-2. ISSN 1872-2067. S2CID 218970877.
- Zhang, Qiaozhi; Wan, Zhonghao; Yu, Iris K. M.; Tsang, Daniel C. W. (2021). "Sustainable production of high-value gluconic acid and glucaric acid through oxidation of biomass-derived glucose: A critical review". Journal of Cleaner Production. 312: 127745. doi:10.1016/j.jclepro.2021.127745. ISSN 0959-6526. S2CID 236243315.
- Current EU approved additives and their E Numbers. Food Standards Agency.
- Julie K. Levy, P. Cynda Crawford, Leslie D. Appel, Emma L. Clifford (2008), Comparison of intratesticular injection of zinc gluconate versus surgical castration to sterilize male dogs. American Journal of Veterinary Research Vol. 69, No. 1, Pages 140–143. doi:10.2460/ajvr.69.1.140
- Ramachandran, V.S.; Lowery, M.S. (1992). "Conduction calorimetric investigation of the effect of retarders on the hydration of Portland cement". Thermochimica Acta. 195: 373–387. doi:10.1016/0040-6031(92)80081-7. ISSN 0040-6031.
- Ma, Suhua; Li, Weifeng; Zhang, Shenbiao; Ge, Dashun; Yu, Jin; Shen, Xiaodong (2015). "Influence of sodium gluconate on the performance and hydration of Portland cement". Construction and Building Materials. 91: 138–144. doi:10.1016/j.conbuildmat.2015.05.068. ISSN 0950-0618.
- Lim, Han Yin; Dolzhenko, Anton V. (2021). "Gluconic acid aqueous solution: A bio-based catalytic medium for organic synthesis". Sustainable Chemistry and Pharmacy. 21: 100443. doi:10.1016/j.scp.2021.100443. ISSN 2352-5541. S2CID 235547468.
- Mycielska, ME; Mohr, MTJ; Schmidt, K; Drexler, K; Rümmele, P; Haferkamp, S; Schlitt, HJ; Gaumann, A; Adamski, J; Geissler, EK (2019). "Potential Use of Gluconate in Cancer Therapy". Frontiers in Oncology. 9: 522. doi:10.3389/fonc.2019.00522. PMC 6593216. PMID 31275855.
- el Saadi M. S., Hall A. H., Hall P. K., Riggs B. S., Augenstein W. L., Rumack B. H. (1989). "Hydrofluoric acid dermal exposure". Vet Hum Toxicol. 31 (3): 243–7. PMID 2741315.
{{cite journal}}: CS1 maint: uses authors parameter (link) - Roblin I., Urban M., Flicoteau D., Martin C., Pradeau D. (2006). "Topical treatment of experimental hydrofluoric acid skin burns by 2.5% calcium gluconate". J Burn Care Res. 27 (6): 889–94. doi:10.1097/01.BCR.0000245767.54278.09. PMID 17091088. S2CID 3691306.
{{cite journal}}: CS1 maint: uses authors parameter (link) - D. Thomas, U. Jaeger, I. Sagoschen, C. Lamberti and K. Wilhelm (2009), Intra-Arterial Calcium Gluconate Treatment After Hydrofluoric Acid Burn of the Hand. CardioVascular and Interventional Radiology, Volume 32, Number 1, pages 155–158 doi:10.1007/s00270-008-9361-1
- Paul Reznikoff and Walther F. Goebel (1937), The preparation of ferrous gluconate and its use in the treatment of hypochromic anelia in rats. Journal of Pharmacology and Experimental Therapy, volume 59 issue 2, page 182.