Poseltinib
Poseltinib (HM71224, LY3337641) is an experimental Bruton's tyrosine kinase inhibitor for the treatment of rheumatoid arthritis. It was developed by Hanmi Pharmaceutical and licensed to Eli Lilly.[1]
 | |
| Clinical data | |
|---|---|
| Other names | HM-7122; LY 3337641 |
| Identifiers | |
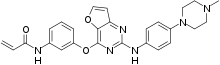
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| UNII | |
| Chemical and physical data | |
| Formula | C26H26N6O3 |
| Molar mass | 470.533 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
Phase II clinical trials began in August 2016 in patients with rheumatoid arthritis. Additional phase II trials are planned for treatment of lupus, lupus nephritis, Sjögren's syndrome, and other immunological conditions.[2]
References
- Alternative Names: HM 71224; LY 3337641. "Poseltinib - AdisInsight". Adisinsight.springer.com. Retrieved 2017-05-22.
{{cite web}}: CS1 maint: multiple names: authors list (link) - "Lilly and Hanmi Announce an Exclusive License and Collaboration Agreement for the Development and Commercialization of an Immunological Therapy (NYSE:LLY)". Investor.lilly.com. 2015-03-19. Retrieved 2017-05-22.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.