Halicin
Halicin (SU-3327) is a chemical compound that acts as an inhibitor of the enzyme c-Jun N-terminal kinase (JNK).[1][2][3] Originally, it was researched for the treatment of diabetes,[4] but development was discontinued for this application due to poor results in testing.
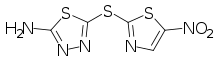 | |
| Clinical data | |
|---|---|
| Trade names | Halicin |
| Legal status | |
| Legal status | |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C5H3N5O2S3 |
| Molar mass | 261.3 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Naming
Researchers named the molecule after the fictional artificial intelligence system from 2001: A Space Odyssey because of how AI predictive computer models were used to identify the probability it would work as an antibiotic.
Antibiotic ability
Halicin was identified by artificial intelligence researchers at the MIT Jameel Clinic in 2019 using an in silico deep learning approach, as a likely broad-spectrum antibiotic.[5][6] This likelihood was verified by in vitro cell culture testing, followed by in vivo tests in mice.[7] It showed activity against drug-resistant strains of Clostridiodes difficile, Acinetobacter baumannii, and Mycobacterium tuberculosis, with an unusual mechanism of action involving the sequestration of iron inside the bacterial cells, that thereby interferes with their ability to regulate the pH balance across the cell membrane properly. Since this is a different mode of action from most antibiotics, halicin retained activity against bacterial strains resistant to many commonly used drugs.[8]
Preliminary studies suggest that halicin kills bacteria by disrupting their ability to maintain an electrochemical gradient across their cell membranes. This gradient is necessary, among other functions, to produce ATP (molecules that cells use to store and transfer energy), so if the gradient breaks down, the cells die. This type of killing mechanism could be difficult for bacteria to develop resistance to.[6]
References
- Augustine C, Cepinskas G, Fraser DD (August 2014). "Traumatic injury elicits JNK-mediated human astrocyte retraction in vitro". Neuroscience. 274: 1–10. doi:10.1016/j.neuroscience.2014.05.009. PMID 24838066. S2CID 9498486.
- Jang S, Javadov S (2014). "Inhibition of JNK aggravates the recovery of rat hearts after global ischemia: the role of mitochondrial JNK". PLOS ONE. 9 (11): e113526. Bibcode:2014PLoSO...9k3526J. doi:10.1371/journal.pone.0113526. PMC 4244102. PMID 25423094.
- Jang S, Yu LR, Abdelmegeed MA, Gao Y, Banerjee A, Song BJ (December 2015). "Critical role of c-jun N-terminal protein kinase in promoting mitochondrial dysfunction and acute liver injury". Redox Biology. 6: 552–564. doi:10.1016/j.redox.2015.09.040. PMC 4625008. PMID 26491845.
- De SK, Stebbins JL, Chen LH, Riel-Mehan M, Machleidt T, Dahl R, et al. (April 2009). "Design, synthesis, and structure-activity relationship of substrate competitive, selective, and in vivo active triazole and thiadiazole inhibitors of the c-Jun N-terminal kinase". Journal of Medicinal Chemistry. 52 (7): 1943–52. doi:10.1021/jm801503n. PMC 2667321. PMID 19271755.
- "Artificial Intelligence Yields New Antibiotic". The MIT Campaign for a Better World. Retrieved 2020-11-13.
- Trafton A (February 20, 2020). "Artificial intelligence yields new antibiotic". MIT News Office.
- Sample I (February 20, 2020). "Powerful antibiotic discovered using machine learning for first time". The Guardian.
- Stokes JM, Yang K, Swanson K, Jin W, Cubillos-Ruiz A, Donghia NM, et al. (February 2020). "A Deep Learning Approach to Antibiotic Discovery". Cell. 180 (4): 688–702.e13. doi:10.1016/j.cell.2020.01.021. PMC 8349178. PMID 32084340.